Evaluation of Abnormal Liver Function Tests J K Limdi, G M Hyde
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

Journal of Blood Group Serology and Molecular Genetics Volume 34, Number 1, 2018 CONTENTS
Journal of Blood Group Serology and Molecular Genetics VOLUME 34, N UMBER 1, 2018 This issue of Immunohematology is supported by a contribution from Grifols Diagnostics Solutions, Inc. Dedicated to advancement and education in molecular and serologic immunohematology Immunohematology Journal of Blood Group Serology and Molecular Genetics Volume 34, Number 1, 2018 CONTENTS S EROLOGIC M ETHOD R EVIEW 1 Warm autoadsorption using ZZAP F.M. Tsimba-Chitsva, A. Caballero, and B. Svatora R EVIEW 4 Proceedings from the International Society of Blood Transfusion Working Party on Immunohaematology Workshop on the Clinical Significance of Red Blood Cell Alloantibodies, Friday, September 2, 2016, Dubai A brief overview of clinical significance of blood group antibodies M.J. Gandhi, D.M. Strong, B.I. Whitaker, and E. Petrisli C A S E R EPORT 7 Management of pregnancy sensitized with anti-Inb with monocyte monolayer assay and maternal blood donation R. Shree, K.K. Ma, L.S. Er and M. Delaney R EVIEW 11 Proceedings from the International Society of Blood Transfusion Working Party on Immunohaematology Workshop on the Clinical Significance of Red Blood Cell Alloantibodies, Friday, September 2, 2016, Dubai A review of in vitro methods to predict the clinical significance of red blood cell alloantibodies S.J. Nance S EROLOGIC M ETHOD R EVIEW 16 Recovery of autologous sickle cells by hypotonic wash E. Wilson, K. Kezeor, and M. Crosby TO THE E DITOR 19 The devil is in the details: retention of recipient group A type 5 years after a successful allogeneic bone marrow transplant from a group O donor L.L.W. -

Liver Scintigrams Compared with Alkaline Phosphatase and Bsp Determinations in the Detection of Metastatic Carcinoma
LIVER SCINTIGRAMS COMPARED WITH ALKALINE PHOSPHATASE AND BSP DETERMINATIONS IN THE DETECTION OF METASTATIC CARCINOMA Satish G. Jhingran, Leon Jordan, Monroe F. Jahns, and Thomas P. Haynie The University of Texas M. D. Anderson Hospital and Tumor Institute at Houston, Houston, Texas Liver scanning is a well-established technique in fling and color scanning devices with a 3 x 2-in. the detection of metastatic liver cancer (1—5), but NaI(Tl) crystal, a 19-hole focusing collimator with from time to time reports are published which ques dot factor and scanning speed adjusted according to tion the accuracy of the scintigram compared with the maximum counting rate (Picker Magnascanner). liver function tests for detecting hepatic metastases In general, only anterior views were obtained. (6) . However, liver function tests often indicate Technetium-99m liver scanning. With no prior abnormality in patients with cancer where no liver preparation, each patient received intravenously 3 metastases are present (2,7). mCi of O9mTc..sulphurcolloid. Scans were performed To assess our own experience in regard to liver 15 mm after injection using a commercially avail scintigrams and liver function tests, a retrospective able gamma camera with an 11 X ½-in.NaI(Tl) study was made comparing the diagnostic accuracy crystal, 4,000 channel straight-bore collimator, and of liver scintigrams with alkaline phosphatase (AP) oscilloscope display with Polaroid recording (Nu and bromsuiphalein (BSP) determinations in a clear-Chicago, Pho/Gamma III). Routinely, an seriesof cancer patients studied in our institution. tenor, right lateral, and posterior scans were per formed on each patient. METHODS Classification of patients. -

Comparison of Histopathology, Immunofluorescence, and Serology
Global Dermatology Cae Report ISSN: 2056-7863 Comparison of histopathology, immunofluorescence, and serology for the diagnosis of autoimmune bullous disorders: an update Seline Ali E1, Seline Lauren N1, Sokumbi Olayemi1* and Motaparthi Kiran2,3 1Department of Dermatology, Medical College of Wisconsin, Milwaukee, WI, USA 2Dermatopathology, Miraca Life Sciences, USA 3Department of Dermatology, University of Texas Southwestern Medical Center, Dallas, TX, USA Introduction In an ELISA, the target antigen of interest (such as the NC16a domain of BP180) is immobilized by physical adsorption or by The diagnosis of autoimmune bullous disorders (AIBDs) relies on antibody capture. When antibody capture is utilized, this is referred to several different diagnostic methods. These include histopathology, as “sandwich ELISA” because the target antigen is bound between the direct immunofluorescence (DIF), indirect immunofluorescence immobilizing antibody and the primary antibody. Primary antibodies (IIF), enzyme-linked immunosorbent assay (ELISA) and are present in the patient’s serum. Enzyme-linked secondary antibodies immunoblotting. When faced with a presumptive AIBD, the are then added which bind the Fc region of primary antibodies. most widely employed method for diagnosis by dermatologists is Substrate is added and converted by the enzyme into a signal. A a combination of histopathology and DIF. While DIF is still the resulting color change, fluorescence, or electrochemical signal is diagnostic method of choice for linear IgA bullous disease and IgA quantitatively measured and reported [5]. pemphigus, ELISA is a more accurate, cost-effective and less invasive method of diagnosis for several AIBDs including pemphigus vulgaris Western blot is synonymous with immunoblot. For this method, and foliaceus, based on currently available evidence [1-3]. -

Review of Intra-Arterial Therapies for Colorectal Cancer Liver Metastasis
cancers Review Review of Intra-Arterial Therapies for Colorectal Cancer Liver Metastasis Justin Kwan * and Uei Pua Department of Vascular and Interventional Radiology, Tan Tock Seng Hospital, Singapore 388403, Singapore; [email protected] * Correspondence: [email protected] Simple Summary: Colorectal cancer liver metastasis occurs in more than 50% of patients with colorectal cancer and is thought to be the most common cause of death from this cancer. The mainstay of treatment for inoperable liver metastasis has been combination systemic chemotherapy with or without the addition of biological targeted therapy with a goal for disease downstaging, for potential curative resection, or more frequently, for disease control. For patients with dominant liver metastatic disease or limited extrahepatic disease, liver-directed intra-arterial therapies including hepatic arterial chemotherapy infusion, chemoembolization and radioembolization are alternative treatment strategies that have shown promising results, most commonly in the salvage setting in patients with chemo-refractory disease. In recent years, their role in the first-line setting in conjunction with concurrent systemic chemotherapy has also been explored. This review aims to provide an update on the current evidence regarding liver-directed intra-arterial treatment strategies and to discuss potential trends for the future. Abstract: The liver is frequently the most common site of metastasis in patients with colorectal cancer, occurring in more than 50% of patients. While surgical resection remains the only potential Citation: Kwan, J.; Pua, U. Review of curative option, it is only eligible in 15–20% of patients at presentation. In the past two decades, Intra-Arterial Therapies for Colorectal major advances in modern chemotherapy and personalized biological agents have improved overall Cancer Liver Metastasis. -

Online-Only Appendix: List of Inclusion and Exclusion Criteria
ONLINE-ONLY APPENDIX: LIST OF INCLUSION AND EXCLUSION CRITERIA INCLUSION CRITERIA [1] Male subjects between the ages of 35 and 70 years, inclusive, with Type 2 diabetes mellitus as defined by the American Diabetes Association (Diabetes care, Vol. 21: S5- S19, 1998) for more than one year. [2] Subjects must have Body Mass Index (BMI) < 36 kg/m². [3] Stable glycemic control (Hb A1C <11%). [4] Subjects must be off all oral hypoglycemic agents 24 hours prior to each study dosing day and off any investigational drug for at least four weeks. [5] Subjects must refrain from strenuous physical activity beginning 72 hours prior to admission and through the duration of the study. [6] Subjects must be willing and able to be confined to the Clinical Research Unit as required by the protocol. [7] Subjects must be willing and able to provide written informed consent. EXCLUSION CRITERIA [1] History or presence of clinically significant cardiovascular, respiratory, hepatic, renal, gastrointestinal, neurological or infectious disorders capable of altering the absorption, metabolism or elimination of drugs, or of constituting a risk factor when taking the study medication. [2] Subjects with gastroparesis, orthostatic hypotension and hypoglycemia unawareness (autonomic neuropathy). [3] Subjects with “brittle” diabetes or predisposition to severe hypoglycemia, e.g., 2 or more serious hypoglycemic episodes (requiring another’s assistance) within the past year, or any hospitalization or emergency room visit due to poor diabetic control within the past 6 months. [4] Evidence of significant active hematological disease and/or cumulative blood donation of 1 unit (500 mL) or more including blood drawn during clinical trials in the last 3 months. -

Impact of Pharmaceutical Prophylaxis on Radiation-Induced Liver Disease Following Radioembolization
cancers Article Impact of Pharmaceutical Prophylaxis on Radiation-Induced Liver Disease Following Radioembolization Max Seidensticker 1,*,†, Matthias Philipp Fabritius 1,*,† , Jannik Beller 2, Ricarda Seidensticker 1, Andrei Todica 3 , Harun Ilhan 3, Maciej Pech 2 , Constanze Heinze 2, Maciej Powerski 2, Robert Damm 2, Alexander Weiss 2, Johannes Rueckel 1 , Jazan Omari 2, Holger Amthauer 4 and Jens Ricke 1 1 Department of Radiology, University Hospital, LMU Munich, Marchioninistr. 15, 81377 Munich, Germany; [email protected] (R.S.); [email protected] (J.R.); [email protected] (J.R.) 2 Klinik für Radiologie und Nuklearmedizin, Otto-von-Guericke Universitätsklinikum, 39120 Magdeburg, Germany; [email protected] (J.B.); [email protected] (M.P.); [email protected] (C.H.); [email protected] (M.P.); [email protected] (R.D.); [email protected] (A.W.); [email protected] (J.O.) 3 Department of Nuclear Medicine, University Hospital, LMU Munich, Marchioninistr. 15, 81377 Munich, Germany; [email protected] (A.T.); [email protected] (H.I.) 4 Department of Nuclear Medicine, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Augustenburger Platz 1, 13353 Berlin, Germany; [email protected] * Correspondence: [email protected] (M.S.); [email protected] (M.P.F.) Citation: Seidensticker, M.; Fabritius, † These authors contributed equally to this work. M.P.; Beller, J.; Seidensticker, R.; Todica, A.; Ilhan, H.; Pech, M.; Simple Summary: Radioembolization has failed to prove survival benefit in randomized trials, and, Heinze, C.; Powerski, M.; Damm, R.; depending on various factors including tumor biology, response rates may vary considerably. -

Application of Quantitative Immunofluorescence to Clinical Serology: Antibody Levels of Treponema Pallidum GRACE L
JOURNAL OF CLINICAL MICROBIOLOGY, May 1992, p. 1294-1296 Vol. 30, No. 5 0095-1137/92/051294-03$02.00/0 Copyright C 1992, American Society for Microbiology Application of Quantitative Immunofluorescence to Clinical Serology: Antibody Levels of Treponema pallidum GRACE L. PICCIOLO* AND DAVID S. KAPLAN Center for Devices and Radiological Health, Food and Drug Administration, 12200 Wilkins Avenue, Rockville, Maryland 20852 Received 27 March 1991/Accepted 20 January 1992 A previously reported method of quantitative immunofluorescence, employing a calibrated photometric system and chemically stabilized fluorescence intensity, was used to replace the subjective, visual method of endpoint determination with a quantitative, calibrated measurement of antibodies to Treponema paUlidum in serum. The results of the quantitative immunofluorescence method showed a 90% correlation with the subjective determinations of the visual method. A quantitative immunofluorescence (QIF) method to de- measured by using an acridine orange filter set, with a 400- to termine immunofluorescence with a quantitative, calibrated 440-nm excitation and LP 470 emission filters. photometric intensity of the fluorescent reaction product has Reducing agent. Dithioerythritol (DTE) (Sigma Chemical been reported previously by us (4). This method used uranyl Co., St. Louis, Mo.) was prepared as stock solution contain- glass slides in the calibration and standardization of the ing 0.3 M reducing agent in 0.5 M Tris buffer, pH 8.2 (Sigma microscope-photometer voltage measurements. To stabilize Chemical Co.). DTE was diluted 1:9 with standard buffered the fluorescence emission, dithioerythritol (DTE) was incor- glycerol mounting medium (Clinical Sciences, Inc., Whip- porated into the buffered glycerol mounting medium of the pany, N.J.) (4, 8). -

Liver Metastases from Colorectal Cancer: Regional Intra-Arterial Treatment Following Failure of Systemic Chemotherapy
British Journal of Cancer (2001) 85(4), 504–508 © 2001 Cancer Research Campaign doi: 10.1054/ bjoc.2001.1972, available online at http://www.idealibrary.com on IIH ii http://www.bjcancer.com Liver metastases from colorectal cancer: regional intra-arterial treatment following failure of systemic chemotherapy A Cyjon1, M Neuman-Levin2, E Rakowsky1, F Greif3, A Belinky2, E Atar2, R Hardoff4, B Brenner1 and A Sulkes1 1Institute of Oncology, Departments of 2Diagnostic Radiology, Unit of Invasive Radiology, 3Surgery B, and 4Nuclear Medicine, Rabin Medical Center, Beilinson Campus, Petah Tiqva 49 100 and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel Summary This study was designed to determine response rate, survival and toxicity associated with combination chemotherapy delivered intra-arterially to liver in patients with hepatic metastases of colorectal origin refractory to standard systemic treatment. A total of 28 patients who failed prior systemic treatment with fluoropyrimidines received a median of 5 cycles of intra-arterial treatment consisting of 5-fluorouracil 700 mg/m2/d, leucovorin 120 mg/m2/d, and cisplatin 20 mg/m2/d for 5 consecutive days. Cycles were repeated at intervals of 5–6 weeks. A major response was achieved in 48% of patients: complete response in 8% and partial response in 40%. The median duration of response was 11.5 months. Median survival was 12 months at a median follow up of 12 months. On multivariate analysis, the only variables with a significant impact on survival were response to treatment and performance status. Toxicity was moderate: grades III–IV neutropenia occurred in 29% of patients. -

Liver Function Tests in Type 2 Sudanese Diabetic Patients
International Journal of Nutrition and Metabolism Vol. 3(2) pp. 17-21, February 2011 Available online http://www.academicjournals.org/ijnam ISSN 2141-2340 ©2011 Academic Journals. Full Length Research Paper Liver function tests in type 2 Sudanese diabetic patients Ayman S. Idris 1*, Koua Faisal Hammad Mekky 1, Badr Eldin Elsonni Abdalla 2 and Khalid Altom Ali 3 1Department of Biochemistry, Faculty of Science and Technology, Al-Neelain University, P. O. Box 12702, Al Baladya St. Khartoum, Sudan. 2Department of Biochemistry, Faculty of Medicine, University of Gezira, Sudan. 3Department of Biochemistry, Faculty of Medicine, International University of Africa, Sudan. Accepted 21 January, 2011 This study was planned to evaluate the liver function in patients with type 2 diabetes mellitus by measuring aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), total protein and albumin. The study was carried out in Wad Medani, Abo Agla Diabetes Centre. 50 patients with type 2 diabetes mellitus (23 male and 27 female) were included in the study. Their ages ranged between 43 and 79 years. Thirty matched normal individuals were taken as control group. In the present study, mean values of ALT, AST and GGT were significantly higher in patients than in the control (P<0.001). Total protein and albumin concentrations in patients were lower compared to control group (P<0.01). The mean of serum glucose in patients revealed significant difference (P<0.000) in comparison to the control group. Although the differences were statistically significant, the means of ALT, AST, GGT, total protein and albumin were falling within the normal range. -
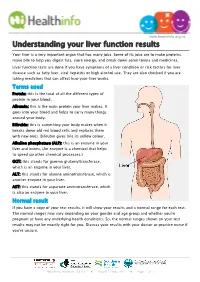
Understanding Your Liver Function Results
www.healthinfo.org.nz Understanding your liver function results Your liver is a very important organ that has many jobs. Some of its jobs are to make proteins, make bile to help you digest fats, store energy, and break down some toxins and medicines. Liver function tests are done if you have symptoms of a liver condition or risk factors for liver disease such as fatty liver, viral hepatitis or high alcohol use. They are also checked if you are taking medicines that can affect how your liver works. Terms used Protein: this is the total of all the different types of protein in your blood. Albumin: this is the main protein your liver makes. It goes into your blood and helps to carry many things around your body. Bilirubin: this is something your body makes when it breaks down old red blood cells and replaces them with new ones. Bilirubin gives bile its yellow colour. Alkaline phosphatase (ALP): this is an enzyme in your liver and bones. (An enzyme is a chemical that helps to speed up other chemical processes.) GGT: this stands for gamma glutamyltransferase, which is an enzyme in your liver. ALT: this stands for alanine aminotransferase, which is another enzyme in your liver. AST: this stands for aspartate aminotransferase, which is also an enzyme in your liver. Normal result If you have a copy of your test results, it will show your results and a normal range for each test. The normal ranges may vary depending on your gender and age group and whether you're pregnant or have any underlying health conditions. -

Clinical Placements Serology Screening for Students
Gawler Place Medical Practice Level 1, Key Invest Building 49 Gawler Place Adelaide SA 5000 T: 08 8212 7175 F: 08 8212 1993 E: [email protected] www.adelaideunicare.com.au CLINICAL PLACEMENTS SEROLOGY SCREENING FOR STUDENTS Gawler Place Medical Practice have put together this Question and Answer sheet to assist you in meeting the requirements of SA Health’s Immunisation for Health Care Workers in South Australia Policy – August 2017. It is a requirement that all Health Care Workers have adequate immunisation against certain diseases prior to working in a clinical environment. What is Serology Screening and why do I need to do it? Serology Screening is a blood test that looks for antibodies in your blood. The purpose of such a test is to detect serum antibodies to prove protective immunity against a disease. Where can I have Serology Screening done? Gawler Place Medical Practice offer Serology Screening appointments to allow incoming students to have their serology screening completed, and where required, obtain their required vaccinations to meet SA Health’s Immunisation for Health Care Workers in South Australia – August 2017. Australian Clinical Labs are located within our Practice so that you can have your blood taken (if required) as soon as you have seen the doctor. How do I make an appointment? You may contact the practice to organise an appointment, our telephone number is 8212 7175 or make a booking online. Please make a long appointment booking. If you need to change or cancel an appointment please contact our Practice on (08) 2127175 as soon as possible so that your appointment can be offered to another person.