Fuel Property Impact on a Premixed Diesel Combustion Mode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigation of Mixture Formation and Combustion in an Ethanol Direct Injection Plus Gasoline Port Injection (EDI+GPI) Engine
Investigation of Mixture Formation and Combustion in an Ethanol Direct Injection plus Gasoline Port Injection (EDI+GPI) Engine By Yuhan Huang A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy School of Electrical, Mechanical and Mechatronic Systems Faculty of Engineering and Information Technology University of Technology Sydney December 2016 Certificate of Original Authorship This thesis is the result of a research candidature conducted jointly with another university as part of a collaborative doctoral degree. I certify that the work in this thesis has not previously been submitted for a degree nor has it been submitted as part of requirements for a degree except as part of the collaborative doctoral degree and/or fully acknowledged within the text. I also certify that the thesis has been written by me. Any help that I have received in my research work and the preparation of the thesis itself has been acknowledged. In addition, I certify that all information sources and literature used are indicated in the thesis. Signature of Student: Date: i Acknowledgements To pursue a doctoral degree could be a long and challenging journey. Through this journey, I fortunately received help and support from the following wonderful people who made this journey enjoyable and fruitful. First of all, I would like thank my principle supervisor Associate Professor Guang Hong who provided huge support and guidance. She invested numerous efforts in supervising me and always cared about my progress and future career. The experience I have acquired and research training I have received from her will greatly benefit my research career. -

Zenith Replacement Carburetors
not print a list price for their carburetors, each dealer can set Zenith Replacement their own prices. Check a few dealers to see who has the bet- Carburetors ter price. These referenced Zenith part numbers were supplied by by Phil Peters Mike Farmer, Application Engineer at Zenith in Bristol, VA, s a follow up to the recent reprinting of the Special where the factory is now located. The Zeniths are a newer Interest Auto article from 1977 (Fall & Winter, 2015) design universal carburetor which means they have multiple Aand the continuing requests for modern replacement fuel, choke, and throttle hook up locations. In addition, there carburetors, I have assembled the following chart of all our are air horn adaptors for modern air filters. They are com- Durant/Star/Flint/etc. engines with corresponding specifica- patible with modern ethanol fuels, have a robust inlet port, tions and replacement Zenith part numbers. As pointed out multiple venturi sizes and “back suction economizer” for part by Norm Toone and other knowledgeable members, there throttle fuel economy. were a number of errors concerning the model numbers and OEM brands in the SIA article. This chart represents The models that were picked for our engines were de- the collective input from a number of helpful members and termined by maximum air flow at 2,200 RPM for the 2 3/8” was done with engine model numbers. Car models were mounts (SAE size #1) and 2,600 RPM for the 2 11/16” not always related to calendar years. Differences in produc- mounts (SAE size #2). -

1. the Future for Automotive Two-Stroke Engines – Part 2
N0.21 THE FUTURE FOR AUTOMOTIVE TWO-STROKE ENGINES (Part 2) CURRENT DEVELOPMENTS Synthetic-based formulation likely to be the preferred option. In the previous issue of Lube, the origins and initial development of the 2T Tendency for combustion chamber deposit formation would have required a engine were described. The earlier engines, characterised by smoky exhausts low ash or ash less lubricant. and poor specific fuel economy, will be remembered by many of the older High temperatures require strong anti-oxidancy. fraternity, as they provided motive power for many commuter lightweight motorcycles, mopeds and scooters in the prewar and postwar years. Many WET SUMP vehicle manufacturers in the UK used proprietary power units manufactured Again, no valve train therefore no requirement for e.g. ZDDP anti-wear by specialist engine manufacturers such as Villiers, who dominated the agent. market, although smaller engine suppliers included companies such as British Diluent unnecessary/undesirable. Anzani. A number of other companies, including Scott, Associated Conventional base oil plus viscosity index-improver is possible (SAE 10/30?). Motorcycles, Excelsior and latterly Ariel also designed their own engines, use Polymer deposits may favour synthetic approach. of which was restricted in the main to vehicles of their own manufacture. However, with the demise of the UK motorcycle industry, 2T engine Tendency for combustion chamber deposits requires a low ash approach. developments, which have progressed steadily during subsequent years, The conclusion was that, in spite of the substantial differences between the have been largely attributable to overseas manufacturers with assistance types of the Orbital engines described above, it may well have been possible from some UK specialist consultancy organizations such as Ricardos at to meet the requirements of both types of engine with a single lubricant, Shoreham. -

The Trilobe Engine Project Greensteam
The Trilobe Engine Project Greensteam Michael DeLessio 4/19/2020 – 8/31/2020 Table of Contents Introduction ................................................................................................................................................... 2 The Trilobe Engine ................................................................................................................................... 2 Computer Design Model ............................................................................................................................... 3 Research Topics and Design Challenges ...................................................................................................... 4 Two Stroke Engines .................................................................................................................................. 4 The Trilobe Cam ....................................................................................................................................... 5 The Flywheel ............................................................................................................................................ 6 Other “Tri” Cams ...................................................................................................................................... 7 The Tristar ............................................................................................................................................. 8 The Asymmetrical Trilobe ................................................................................................................... -

BOSE , CUTTACK 1. Mist Lubrication System
BOSE , CUTTACK CHAPTER-06 LUBRICATION SYSTEM IN AUTOMOBILE Functions of lubricating oil: A good lubricating oil should perform the following function. · It reduces the friction between the moving parts. · It cools the piston so it also acts as a cooling medium. · It also prevents the leakage of gas between the piston and cylinder because it makes a film of lubricant between them. · It also reduces the noise between the rubbing surfaces. The various lubrication systems used for lubricating the various parts of engine are classified as 1. Mist lubrication system 2. Wet sump lubrication system, and 3. Dry sump lubrication system. 1. Mist lubrication system: Mist lubrication system is a very simple type of lubrication. In this system, the small quantity of lubricating oil (usually 2 to 3%) is mixed with the fuel (preferably gasoline). The oil and fuel mixture is introduced through the carburetor. The gasoline vaporized and oil in the form of mist enters the cylinder via the crank base. The droplets of oil strike the crank base. The droplets of oil strike the crank base, lubricate the main and connecting rod bearings and the rest of the oil lubricates the piston, piston rings and cylinder. The system is preferred in two stroke engines where crank base lubrication is not required. In a two-stroke engine, the charge is partially compressed in a crank base, so it is not possible to have the oil in the crank base. This system is simple, low cost and maintenance free because it does not require any oil pump, filter, etc. However, it has certain serious disadvantages. -

PICTURE DESCRIPTION REPLACEMENT MANUFACTURER & P/N Power Steering Bracket Assembly 97-253BK
REPLACEMENT PICTURE DESCRIPTION MANUFACTURER & P/N 97-253BK (Black - LT4 for Add-On P/ S to Dry Sump ONLY) Power Steering Bracket Assembly 97-251BK (Black Dry Sump) Manifold Assembly, Water Pump Alternator Bracket Holley 97-254 Alternator Bracket Spacer Holley 97-255 (Wet Sump) 97-262BK (Black Wet Sump) Spacer, P/S Bracket Assembly Gaskets, Water Pump GM 12657430 Water Pump Drive Assembly -Clockwise Rotation- Holley 97-245 (not included with wet sump applications) Water Pump Assembly Gasket GM 12619770 REPLACEMENT PICTURE DESCRIPTION MANUFACTURER & P/N Bando 6PK1780 (20-220, Belt (6-Rib), Accessories 20-221, 20-230 & BK versions) Bando 6PK1500 (20-224 & 20-224) Gates FleetRunner® K080820HD Belt (8-Rib), Supercharger (20-220, 20-221, 20-222 & BK ver.) Bando 8PK2130 (20-223 & BK ver.) Bando 11PK2185 (20-233 & BK ver.) Belt (11-Rib), Supercharger Bando Aramid 11PK2137A (20-230 & BK ver.) Holley 97-247 / Thermostat 190° & Housing GM 12674634 LT4 - Holley 97-243 / GM 12663624 Supercharger Tensioner Holley 97-244 / Accessory Tensioner GM 12669076 LT4 - Holley 97-242 / GM 12642706 Supercharger Smooth Idler, Lower LT4 - Holley 97-241 / GM 12665845 Supercharger Smooth Idler, Center LT4 - Holley 97-240 / GM 12678245 Supercharger Ribbed Idler, Center Holley 97-249 / Accessories Smooth Idler Gates 36101 Alternator Harness Pigtail Holley 197-400 197-303 (Black) Alternator REPLACEMENT PICTURE DESCRIPTION MANUFACTURER & P/N Adapter Manifold for A/C Compressor & Holley 199-201 / 199-201BK Hardware (Long Option) Adapter Manifold for A/C Compressor & Holley -

Estimation of Fuel Economy Improvement in Gasoline Vehicle Using Cylinder Deactivation
energies Article Estimation of Fuel Economy Improvement in Gasoline Vehicle Using Cylinder Deactivation Nankyu Lee 1 , Jinil Park 1,*, Jonghwa Lee 1 , Kyoungseok Park 2, Myoungsik Choi 3 and Wongyu Kim 3 1 Department of Mechanical Engineering, Ajou University, Suwon 16499, Gyeonggi, Korea; [email protected] (N.L.); [email protected] (J.L.) 2 Department of Mechanical System Engineering, Kumho National Institute of Technology, Gumi 39177, Gyeongbuk, Korea; [email protected] 3 Hyundai Motor Company, 150, Hyundaiyeonguso-ro, Jangdeok-ri, Namyang-eup, Hwaseong-si 18280, Gyeonggi-do, Korea; [email protected] (M.C.); [email protected] (W.K.) * Correspondence: [email protected]; Tel.: +82-31-219-2337 Received: 8 October 2018; Accepted: 6 November 2018; Published: 8 November 2018 Abstract: Cylinder deactivation is a fuel economy improvement technology that has attracted particular attention recently. The currently produced cylinder deactivation engines utilize fixed-type cylinder deactivation in which only a fixed number of cylinders are deactivated. As fixed-type cylinder deactivation has some shortcomings, variable-type cylinder deactivation with no limit on the number of deactivated cylinders is under research. For variable-type cylinder deactivation, control is more complicated and production cost is higher than fixed-type cylinder deactivation. Therefore, it is necessary to select the cylinder deactivation control method considering both advantages and disadvantages of the two control methods. In this study, a fuel economy prediction simulation model was created using the measurement data of various vehicles with engine displacements of 1.0–5.0 L. The fuel economy improvement of fixed-type cylinder deactivation was compared with that of variable-type cylinder deactivation using the created simulation. -

Vacuum Pumps Add Easy Horsepower to Any Engine. Here's
The three core components of any vacuum pump system are the pump, drive pulleys, and breather tank. Aerospace Components' billet hardware is too pretty to hide under the hood. The company offers turnkey kits for both small- and big-block Fords. TECH Vacuum Pumps Add Easy Horsepower to Any Engine. BY STEPHEN KIM PHOTOS Here's a Closer Look and How they Work COURTESY OF THE MANUFACTURERS hen a bazillion PSI of cylinder pressure pushes down on the pistons, some of it’s going to leak past the rings and into the crankcase. That’s why old cars have breathers, and newer cars have W PCV systems. While a wee bit of blow-by is completely normal— even in a 100-percent healthy motor—if an engine’s crankcase isn’t ventilated, that wee bit of blow-by is enough to pop oil seals and gaskets in no time. No bueno, homie. So what’s a racer to do? Valve cover breathers are an easy fix for non-emissions engines, but zip-tying rags onto the breathers and using them as disposable “catch cans” is downright ghetto. Instead of venting crankcase fumes into the atmosphere, late-model motors circulate them back into the intake manifold by utilizing a PCV valve. While these closed-loop systems are great for tree-huggers, they also re-circulate tiny oil droplets into the induction tract, which reduces knock resistance and can lead to detonation. Still no bueno, homie. So what’s a racer to do? The ultimate solution is sucking the fumes out of the crankcase with a vacuum pump. -

Flow Testing and Evaluation
About MAX BMW Motorcycles Machine Shop Articles: 2017 brings MAX BMW's Machine Shop to full operational status and a series of articles on our individual machines and operational practices. In this series, we highlight some of the specific equipment, tools and jigs we have developed to come to the exacting standards of ultimate quality, attention to detail, accurate measurements and swift turnaround of customer jobs. ARTICLE 3 April 21, 2017 Flow Testing and Evaluation In this article, we are going to start looking at a few performance services that are available at MAX BMW. Specifically, we’ll look at an overview of cylinder head performance through flow testing and evaluation. With any machine shop work, the proper tools are essential when exacting results and a high standard of quality are required. One of those tools is our Super Flow SF600 cylinder head flow bench. 2 When building an engine where more power is desired, everything in the engine must be considered to be working together as a package. The starting point for any project is always understanding its intended use. Are you building a stock engine, a high horse power street bike or perhaps a track-only bike that sees sustained high rpms and loads? Once this is determined, the details to achieve these goals fall into place. Specifically, engine displacement, cam selection, cylinder head improvements such as valve size and material, valve seat machining and port design are all areas that can be modified and tuned for performance improvements. Even though there have been volumes written on air flow theory and port design, this will be a brief overview to help understand how it can all be measured, modified and tested to prove effective results. -

Air Filter Sizing
SSeeccoonndd SSttrriikkee The Newsletter for the Superformance Owners Group January 17, 2008 / September 5, 2011 Volume 8, Number 1 SECOND STRIKE CARBURETOR CALCULATOR The Carburetor Controlling the airflow introduces the throttle plate assembly. Metering the fuel requires measuring the airflow and measuring the airflow introduces the venturi. Metering the fuel flow introduces boosters. The high flow velocity requirement constrains the size of the air passages. These obstructions cause a pressure drop, a necessary consequence of proper carburetor function. Carburetors are sized by airflow, airflow at 5% pressure drop for four-barrels, 10% for two-barrels. This means that the price for a properly sized four-barrel is a 5% pressure loss and a corresponding 5% horsepower loss. The important thing to remember is that carburetors are sized to provide balanced performance across the entire driving range, not just peak horsepower. When designing low and mid range metering, the carburetor engineers assume airflow conditions for a properly As with everything in the engine, airflow is power. sized carburetor - one sized for 5% loss at the power peak. Carburetors are a key to airflow. As with any component, the key to best all around performance is to pick parts that match Selecting a larger carburetor than recommended will reduce your performance goal and each other – carburetor, intake, pressure losses at high rpm and may help top end horsepower, heads, exhaust, cam, displacement, rpm range, and bottom but will have lower flow velocity at low rpm and poor end. metering and mixing with a loss in low and mid range power and drivability. -

LUBRICATION SYSTEM in IC ENGINES Definition of Lubrication
LUBRICATION SYSTEM IN IC ENGINES Definition of lubrication Lubrication is the action of applying a substance such as oil or grease to an engine or component so as to minimize friction and allow smooth movement. Lubrication System Lubricating system is a mechanical system of lubricating internal combustion engines in which a pump forces oil into the engine bearings. PURPOSE OF LUBRICATION ➢ To reduce the friction between moving parts ➢ To increase the efficiency ➢ To minimize the vibrations ➢ To reduce the corrosion and carbon deposits ➢ To reduce the heat of moving parts ➢ To minimize power loss due to friction ➢ To reduce the noise created by moving parts ➢ To provide cooling to the engine TYPES OF LUBRICANTS ➢SOLID LUBRICANTS ❑ e.g. graphite ,molybdenum ,mica ➢ SEMI-SOLID LUBRICANTS ❑ e.g. heavy greases ➢ LIQUID LUBRICANTS ❑ e.g. mineral oil obtained by refining petroleum. PROPERTIES OF LUBRICANTS ➢ Viscosity ❑ It is a measure of the resistance to flow of an oil ❑ It is measured in saybolt universal seconds (SUS) ❑ It is expressed in centistokes ,centipoises and redwood seconds ➢ Viscosity Index ❑ viscosity of oil decreases with increase in temperature ➢ Cloud point ❑ If an oil is cooled , it will start solidifying at some time . ❑ Temperature at which oil starts solidifying , is called cloud point PROPERTIES OF LUBRICANTS ➢Pour point ❑ It is temperature just above which the oil sample will not flow under certain prescribed conditions ❑ this property is important for operation of engines and substances at low temperature conditions ➢ Flash point and Fire point ❑ The temperature at which vapour of an oil flash when subjected to a naked flame is called flash point ❑ Fire point is the temperature at which the oil ,it once lit with flame ,will burnt steadily at least for 5 seconds ➢ Specific Gravity ❑ It varies between 0.85 to 0.96 SAE Number • Society of Automotive Engineer has recommended SAE viscosity number for lubricating oils. -
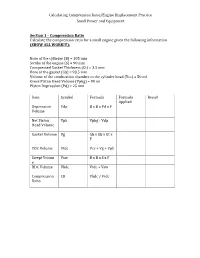
Calculating Compression Ratio/Engine Displacement Practice Small Power and Equipment Secti
Calculating Compression Ratio/Engine Displacement Practice Small Power and Equipment Section 1 – Compression Ratio Calculate the compression ratio for a small engine given the following information (SHOW ALL WORK!!!): Bore of the cylinder (B) = 105 mm Stroke of the engine (S) = 90 mm Compressed Gasket Thickness (Gt) = 3.5 mm Bore of the gasket (Gb) = 93.5 mm Volume of the combustion chamber in the cylinder head (Vcc) = 50 ml Gross Piston Head Volume (Vphg) = 80 ml Piston Depression (Pd) = 25 mm Item Symbol Formula Formula Result Applied Depression Vdp B x B x Pd x F Volume Net Piston Vph Vphg - Vdp Head Volume Gasket Volume Vg Gb x Gb x Gt x F TDC Volume Vtdc Vcc + Vg + Vph Swept Volum Vsw B x B x S x F e BDC Volume Vbdc Vtdc + Vsw Compression CR Vbdc / Vtdc Ratio Calculating Compression Ratio/Engine Displacement Practice Small Power and Equipment Bore of the cylinder (B) = 110 mm Stroke of the engine (S) = 75 mm Compressed Gasket Thickness (Gt) = 1.5 mm Bore of the gasket (Gb) = 80.5 mm Volume of the combustion chamber in the cylinder head (Vcc) = 50 ml Gross Piston Head Volume (Vphg) = 88 ml Piston Depression (Pd) = 15 mm Item Symbol Formula Formula Result Applied Depression Vdp B x B x Pd x F Volume Net Piston Vph Vphg - Vdp Head Volume Gasket Volume Vg Gb x Gb x Gt x F TDC Volume Vtdc Vcc + Vg + Vph Swept Volum Vsw B x B x S x F e BDC Volume Vbdc Vtdc + Vsw Compression CR Vbdc / Vtdc Ratio Section 2 – Engine Displacement (5 points each = 10 points) Calculate the engine displacement given the following information (SHOW ALL WORK!!!).