Effect of Gaps on Species Diversity in the Naturally Regenerated Mixed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GIS Assessment of the Status of Protected Areas in East Asia
CIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, 1. Lysenko, S. Chape, I. May and C. Brown March 2005 IUCN V 9> m The World Conservation Union UNEP WCMC Digitized by the Internet Archive in 20/10 with funding from UNEP-WCMC, Cambridge http://www.archive.org/details/gisassessmentofs05mack GIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, I. Lysenko, S. Chape, I. May and C. Brown March 2005 UNEP-WCMC IUCN - The World Conservation Union The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of UNEP, UNEP-WCMC, and IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. UNEP-WCMC or its collaborators have obtained base data from documented sources believed to be reliable and made all reasonable efforts to ensure the accuracy of the data. UNEP-WCMC does not warrant the accuracy or reliability of the base data and excludes all conditions, warranties, undertakings and terms express or implied whether by statute, common law, trade usage, course of dealings or otherwise (including the fitness of the data for its intended use) to the fullest extent permitted by law. The views expressed in this publication do not necessarily reflect those of UNEP, UNEP-WCMC, and IUCN. Produced by: UNEP World Conservation Monitoring Centre and IUCN, Gland, Switzerland and Cambridge, UK Cffti IUCN UNEP WCMC The World Conservation Union Copyright: © 2005 UNEP World Conservation Monitoring Centre Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. -

The Variations of Land Surface Phenology in Northeast China and Its Responses to Climate Change from 1982 to 2013
remote sensing Article The Variations of Land Surface Phenology in Northeast China and Its Responses to Climate Change from 1982 to 2013 Jianjun Zhao 1,†, Yanying Wang 1,†, Zhengxiang Zhang 1,*, Hongyan Zhang 1,*, Xiaoyi Guo 1,†, Shan Yu 1,2,†, Wala Du 3,† and Fang Huang 1,† 1 School of Geographical Sciences, Northeast Normal University, Changchun 130024, China; [email protected] (J.Z.); [email protected] (Y.W.); [email protected] (X.G.); [email protected] (S.Y.); [email protected] (F.H.) 2 Inner Mongolia Key Laboratory of Remote Sensing and Geographic Information System, Huhhot 010022, China 3 Ecological and Agricultural Meteorology Center of Inner Mongolia Autonomous Region, Huhhot 010022, China; [email protected] * Correspondence: [email protected] (Z.Z.); [email protected] (H.Z.); Tel./Fax: +86-431-8509-9550 (Z.Z.); +86-431-8509-9213 (H.Z.) † These authors contributed equally to this work. Academic Editors: Petri Pellikka, Lars Eklundh, Alfredo R. Huete and Prasad S. Thenkabail Received: 4 February 2016; Accepted: 4 May 2016; Published: 12 May 2016 Abstract: Northeast China is located at high northern latitudes and is a typical region of relatively high sensitivity to global climate change. Studies of the land surface phenology in Northeast China and its response to climate change are important for understanding global climate change. In this study, the land surface phenology parameters were calculated using the third generation dataset from the Global Inventory Modeling and Mapping Studies (GIMMS 3g) that was collected from 1982 to 2013 were estimated to analyze the variations of the land surface phenology in Northeast China at different scales and to discuss the internal relationships between phenology and climate change. -

Tetrao Urogalloides) in Northeast China from 1950 to 2010 Based on Local Historical Documents
Pakistan J. Zool., vol. 48(6), pp. 1825-1830, 2016. Decline and Range Contraction of Black-Billed Capercaillie (Tetrao urogalloides) in Northeast China from 1950 to 2010 Based on Local Historical Documents Yueheng Ren, Li Yang, Rui Zhang, Jiang Lv, Mujiao Huang and Xiaofeng Luan* School of Nature Conservation, Beijing Forestry University, NO.35 Tsinghua East Road Haidian District, Beijing, P. R. China, Beijing, 100083, P. R. China, Yueheng Ren, Li Yang, Rui Zhang contributed equally. A B S T R A C T Article Information Received 21 August 2015 The black-billed capercaillie (Tetrao urogalloides) is a large capercaillie which is considered an Revised 14 March 2016 endangered species that has undergone a dramatic decline throughout the late 20th century. This Accepted 19 May 2016 species is now rare or absent in Northeast China and needs immediate protection. Effective Available online 25 September 2016 conservation and management could be hampered by insufficient understanding of the population decline and range contraction; however, any historical information, whilst being crucial, is rare. In Authors’ Contribution this paper, we present local historical documents as one problem-solving resource for large-scale YR, LY and XL conceived and designed the study. LY, YR, JL and analysis of this endangered species in order to reveal the historical population trend in Northeast MH were involved in data collection. China from 1950 to 2010. Our results show that the population was widely distributed with a large YR, LY and RZ were involved in population in Northeast China before the 1980s. Because of increasing habitat destruction in data processing. -

Sp. Nov. from Northeast China
ISSN (print) 0093-4666 © 2013. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/124.269 Volume 124, pp. 269–278 April–June 2013 Russula changbaiensis sp. nov. from northeast China Guo-Jie Li1,2, Dong Zhao1, Sai-Fei Li1, Huai-Jun Yang3, Hua-An Wen1a*& Xing-Zhong Liu1b* 1State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, No 3 1st Beichen West Road, Chaoyang District, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3 Shanxi Institute of Medicine and Life Science, No 61 Pingyang Road, Xiaodian District, Taiyuan 030006, China Correspondence to *: [email protected], [email protected] Abstract —Russula changbaiensis (subg. Tenellula sect. Rhodellinae) from the Changbai Mountains, northeast China, is described as a new species. It is characterized by the red tinged pileus, slightly yellowing context, small basidia, short pleurocystidia, septate dermatocystidia with crystal contents, and a coniferous habitat. The phylogenetic trees based on ITS1-5.8S- ITS2 rDNA sequences fully support the establishment of the new species. Key words —Russulales, Russulaceae, taxonomy, morphology, Basidiomycota Introduction The worldwide genus ofRussula Pers. (Russulaceae, Russulales) is characterized by colorful fragile pileus, amyloid warty spores, abundant sphaerocysts in a heteromerous trama, and absence of latex (Romagnesi 1967, 1985; Singer 1986; Sarnari 1998, 2005). As a group of ectomycorrhizal fungi, it includes a large number of edible and medicinal species (Li et al. 2010). The genus has been extensively investigated with a long, rich and intensive taxonomic history in Europe (Miller & Buyck 2002). Although Russula species have been consumed in China as edible and medicinal use for a long time, their taxonomy has been overlooked (Li & Wen 2009, Li 2013). -
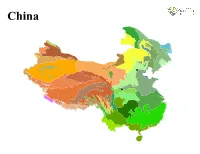
R Graphics Output
China China LEGEND Previously sampled Malaise trap site Ecoregion Alashan Plateau semi−desert North Tibetan Plateau−Kunlun Mountains alpine desert Altai alpine meadow and tundra Northeast China Plain deciduous forests Altai montane forest and forest steppe Northeast Himalayan subalpine conifer forests Altai steppe and semi−desert Northern Indochina subtropical forests Amur meadow steppe Northern Triangle subtropical forests Bohai Sea saline meadow Northwestern Himalayan alpine shrub and meadows Central China Loess Plateau mixed forests Nujiang Langcang Gorge alpine conifer and mixed forests Central Tibetan Plateau alpine steppe Ordos Plateau steppe Changbai Mountains mixed forests Pamir alpine desert and tundra Changjiang Plain evergreen forests Qaidam Basin semi−desert Da Hinggan−Dzhagdy Mountains conifer forests Qilian Mountains conifer forests Daba Mountains evergreen forests Qilian Mountains subalpine meadows Daurian forest steppe Qin Ling Mountains deciduous forests East Siberian taiga Qionglai−Minshan conifer forests Eastern Gobi desert steppe Rock and Ice Eastern Himalayan alpine shrub and meadows Sichuan Basin evergreen broadleaf forests Eastern Himalayan broadleaf forests South China−Vietnam subtropical evergreen forests Eastern Himalayan subalpine conifer forests Southeast Tibet shrublands and meadows Emin Valley steppe Southern Annamites montane rain forests Guizhou Plateau broadleaf and mixed forests Suiphun−Khanka meadows and forest meadows Hainan Island monsoon rain forests Taklimakan desert Helanshan montane conifer forests -

Potential Distribution Shifts of Plant Species Under Climate Change in Changbai Mountains, China
Article Potential Distribution Shifts of Plant Species under Climate Change in Changbai Mountains, China Lei Wang 1, Wen J. Wang 1,*, Zhengfang Wu 2,*, Haibo Du 2, Shengwei Zong 2 and Shuang Ma 1,2 1 Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, China; [email protected] (L.W.); [email protected] (S.M.) 2 Key Laboratory of Geographical Processes and Ecological Security in Changbai Mountains, Ministry of Education, School of Geographical Sciences, Northeast Normal University, Changchun 130024, China; [email protected] (H.D.); [email protected] (S.Z.) * Correspondence: [email protected] (W.J.W.); [email protected] (Z.W.) Received: 18 April 2019; Accepted: 7 June 2019; Published: 11 June 2019 Abstract: Shifts in alpine tundra plant species have important consequences for biodiversity and ecosystem services. However, recent research on upward species shifts have focused mainly on polar and high-latitude regions and it therefore remains unclear whether such vegetation change trends also are applicable to the alpine tundra at the southern edges of alpine tundra species distribution. This study evaluated an alpine tundra region within the Changbai Mountains, China, that is part of the southernmost alpine tundra in eastern Eurasia. We investigated plant species shifts in alpine tundra within the Changbai Mountains over the last three decades (1984–2015) by comparing contemporary survey results with historical ones and evaluated potential changes in the distribution of dwarf shrub and herbaceous species over the next three decades (2016–2045) using a combination of observations and simulations. The results of this study revealed that the encroachment of herbaceous plants had altered tundra vegetation to a significant extent over the last three decades, especially within low and middle alpine tundra regions in Changbai Mountains, China. -

Changes of Major Terrestrial Ecosystems in China Since 1960
Global and Planetary Change 48 (2005) 287–302 www.elsevier.com/locate/gloplacha Changes of major terrestrial ecosystems in China since 1960 Tian Xiang YueT, Ze Meng Fan, Ji Yuan Liu Institute of Geographical Sciences and Natural Resources Research, Chinese Academy of Sciences, Jia No. 11, Datun, Anwai, 100101 Beijing, China Abstract Daily temperature and precipitation data since 1960 are selected from 735 weather stations that are scattered over China. After comparatively analyzing relative interpolation methods, gradient-plus-inverse distance squared (GIDS) is selected to create temperature surfaces and Kriging interpolation method is selected to create precipitation surfaces. Digital elevation model of China is combined into Holdridge Life Zone (HLZ) model on the basis of simulating relationships between temperature and elevation in different regions of China. HLZ model is operated on the created temperature and precipitation surfaces in ARC/ INFO environment. Spatial pattern of major terrestrial ecosystems in China and its change in the four decades of 1960s, 1970s, 1980s and 1990s are analyzed in terms of results from operating HLZ model. The results show that HLZ spatial pattern in China has had a great change since 1960. For instance, nival area and subtropical thorn woodland had a rapid decrease on an average and they might disappear in 159 years and 96 years, respectively, if their areas would decrease at present rate. Alpine dry tundra and cool temperate scrub continuously increased in the four decades and the decadal increase rates are, respectively, 13.1% and 3.4%. HLZ patch connectivity has a continuous increase trend and HLZ diversity has a continuous decrease trend on the average. -

4 Days Tour for Changbai Mountains (Reference Tours at Conference Rate)
4 days tour for Changbai Mountains (Reference tours at Conference Rate) Quotations: Quotations Single supplement 1PAX RMB6950/per person 2-5PAX RMB5300/per person RMB840/per person 6-9PAX RMB3850/per person RMB840/per person Group of 10 persons or more RMB3210/per person RMB780/per person Included: 4 stars hotel(double room),meals marked, transportation, local guide service(speaks both English and Chinese),entry fee of sightseeing, insurance, Dalian—Yianji single trip airfare. Schedule: 6 Jul: Dalian/Yanji (D) Drive to Dalian airport, and fly to Yanji, CZ6621(16:30-19:05), our tour guide will wait for you in Yanji airport, then bus to Erdao Baihe. After dinner, check in hotel, overnight in Erdao Baihe . 7 Jul: Changbai Mountain (B,L,D) After breakfast, Go to the sixth largest famous mountain in China-Changbai Mountain. Then take the environmental protection car or step on the long corridor.(According to the weather condition).Watch the highest volcano lake. Visit the largest drop in the elevation and the highest above sea level volcano waterfall in the world-Changbai waterfall. And distributed in the hot springs of thousands of square meters-gather the spring of dragon (Can wash the hot spring pay by yourself).Visit small heaven lake, green deep pool, valley forest-underground forest. Can taste various of green flavor meal in Changbai Mountain for lunch. Check in hotel, overnight in Erdao Baihe . 8 Jul: Changbai Mountain / Red flag village (B,L,D) After breakfast, go to the first Korean village of China -red flag village(71 km,1.5 hours by bus) .Visit the Koreans’ style houses, watches the show。Learning the Koreans' culture, learn the Koreans' folk dance with the mothers of the Koreans,Make the harvest cakes, meals of the Koreans nationality such as rice cake, laver rice, the pickles, etc. -

Variability in Snow Cover Phenology in China from 1952 to 2010
Hydrol. Earth Syst. Sci., 20, 755–770, 2016 www.hydrol-earth-syst-sci.net/20/755/2016/ doi:10.5194/hess-20-755-2016 © Author(s) 2016. CC Attribution 3.0 License. Variability in snow cover phenology in China from 1952 to 2010 Chang-Qing Ke1,2,6, Xiu-Cang Li3,4, Hongjie Xie5, Dong-Hui Ma1,6, Xun Liu1,2, and Cheng Kou1,2 1Jiangsu Provincial Key Laboratory of Geographic Information Science and Technology, Nanjing University, Nanjing 210023, China 2Key Laboratory for Satellite Mapping Technology and Applications of State Administration of Surveying, Mapping and Geoinformation of China, Nanjing University, Nanjing 210023, China 3National Climate Center, China Meteorological Administration, Beijing 100081, China 4Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters Faculty of Geography and Remote Sensing, Nanjing University of Information Science & Technology, Nanjing 210044, China 5Department of Geological Sciences, University of Texas at San Antonio, Texas 78249, USA 6Collaborative Innovation Center of South China Sea Studies, Nanjing 210023, China Correspondence to: Chang-Qing Ke ([email protected]) Received: 12 February 2015 – Published in Hydrol. Earth Syst. Sci. Discuss.: 30 April 2015 Revised: 11 January 2016 – Accepted: 3 February 2016 – Published: 19 February 2016 Abstract. Daily snow observation data from 672 stations in 1 Introduction China, particularly the 296 stations with over 10 mean snow cover days (SCDs) in a year during the period of 1952–2010, are used in this study. We first examine spatiotemporal vari- Snow has a profound impact on the surficial and atmospheric ations and trends of SCDs, snow cover onset date (SCOD), thermal conditions, and is very sensitive to climatic and en- and snow cover end date (SCED). -

Introduction
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. 8 — introduCtion Introduction China is a magnificent country and one of the most diverse on Earth. its size ranks fourth among the world’s nations (9,596,960 km2), and it is home to over 1.3 billion people. The topography of China ranges from the highest elevation on Earth (Mount Everest, or Chomolungma; 8,850 m) to one of the lowest (Turpan Basin; 154 m below sea level). Chinese environments include some of Earth’s For general queries, contact [email protected] © Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. introduCtion — 9 most extensive and driest deserts (the Taklimakan and Gobi) and its highest pla- teau (the Tibetan Plateau or “Roof of the World”). Habitats range from tropical to boreal forest, and from extensive grasslands to desert (see the habitat images, map 1, and map 2). This wide variety of habitats has contributed greatly to the richness of China’s mammal fauna. Additionally, the geographic location of China, at the suture zone between the Palaearctic and indo-Malayan biogeo- graphic regions, further contributes to the country’s mammal diversity. overall, more than 10 percent of the world’s species of mammals live in China (556/5,416); an additional 29 species of cetaceans live in offshore waters (see Appendix i). -

On the Growth of National Geoparks in China: Distribution, Interpretation, and Regional Comparison
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 1-1-2011 On the growth of national geoparks in China: distribution, interpretation, and regional comparison Guifang Yang China University of Geosciences Zhenghong Chen China University of Geosciences Wuhan Mingzhong Tian China University of Geosciences Fadong Wu China University of Geosciences Robert A. L Wray University of Wollongong, [email protected] See next page for additional authors Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Yang, Guifang; Chen, Zhenghong; Tian, Mingzhong; Wu, Fadong; Wray, Robert A. L; and Ping, Yamin: On the growth of national geoparks in China: distribution, interpretation, and regional comparison 2011, 157-176. https://ro.uow.edu.au/scipapers/4086 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] On the growth of national geoparks in China: distribution, interpretation, and regional comparison Abstract Since the year 2000 China has created 139 National Geoparks; it started under the guidance of the former UNESCO's Division of Earth Science, and has therefore become one of the pioneers in this aspect. Many National Geoparks in China have been described over the past decade, but an understanding of the range of various landform features and their connection with geological and climatic constraints has not previously been published. Based on an increasing awareness of National Geoparks, the aim of this contribution is to provide a comprehensive overview of the National Geoparks of China by reviewing the geological heritage and their intrinsic linkages with geological and climatic controls. -

1. Brief Introduction to the Institute of Geology
-1- The Institute of Geology, Chinese Academy of Geological Sciences (CAGS) Preface The Institute of Geology, Chinese Academy of Geological Sciences (CAGS), is a national public scientific research institution and is mainly engaged in national fundamental, public, strategic and frontier geological survey and geoscientific research. Entering the new century, and in particular during the past 5 years, the Institute has made notable progress in scientific research, personnel training and international cooperation, with increasing cooperation and exchange activities, expanded fields of cooperation, abundant output of new research results, and an increased number of papers published in “Nature”, “Science” and other high-impact international scientific journals. In the light of this new situation and in order to publicize, in a timely manner, annual progress and achievements of the Institute to enhance its international reputation, an English version of the Institute’s Annual Report has been published since 2010. Similar to previous reports, the Annual Report 2015 includes the following 7 parts: (1) Introduction to the Institute of Geology, CAGS; (2) Ongoing Research Projects; (3) Research Achievements and Important Progress; (4) International Cooperation and Academic Exchange; (5) Important Academic Activities in 2015; (6) Postgraduate Education; (7) Publications. In order to avoid confusion in the meaning of Chinese and foreign names, all family names in this Report are capitalized. We express our sincere gratitude to colleagues of related research departments and centers of the Institute for their support and efforts in compiling this Report and providing related material – a written record of the hard work of the Institute’s scientific research personnel for the year 2015.