Multifocal Intraocular Lens Exchange in Patients with Ocular Comorbidities: Indications and Outcomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
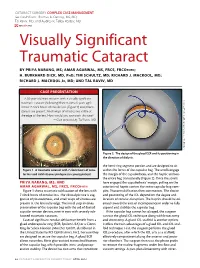
Visually Significant Traumatic Cataract
CATARACT SURGERY COMPLEX CASE MANAGEMENT Section Editors: Thomas A. Oetting, MS, MD; Tal Raviv, MD; and Audrey R. Talley Rostov, MD eyetube.net Visually Significant Traumatic Cataract BY PRIYA NARANG, MS; AMAR AGARWAL, MS, FRCS, FRCOPHTH; H. BURKHARD DICK, MD, PHD; TIM SCHULTZ, MD; RICHARD J. MACKOOL, MD; RICHARD J. MACKOOL JR, MD; AND TAL RAVIV, MD CASE PRESENTATION A 50-year-old man presents with a visually significant (Courtesy of Priya Narang, MS, and Amar Agarwal, FRCS, FRCOphth.) traumatic cataract (following blunt trauma 5 years ago). About 7 clock hours of zonular loss (Figure 1) and phaco- donesis are present. Small wisps of vitreous are visible at the edge of the lens. How would you approach this case? —Case prepared by Tal Raviv, MD. (Courtesy of Tal Raviv, MD.) Figure 2. The design of the glued ECR and its positioning in the direction of dialysis. the hemi-ring segment portion and are designed to sit Figure 1. A traumatic cataract with 7 clock hours of zonu- within the fornix of the capsular bag. The scrolls engage lar loss and mild vitreous prolapse in a young patient. the margin of the capsulorhexis, and the haptic anchors the entire bag transsclerally (Figure 2). Once the scrolls PRIYA NARANG, MS, AND have engaged the capsulorhexis’ margin, pulling on the AMAR AGARWAL, MS, FRCS, FRCOPHTH exteriorized haptic centers the entire capsular bag com- Figure 1 shows traumatic subluxation of the lens with plex. Phacoemulsification then commences. The choice 7 clock hours of zonular loss. The clinical picture is sug- and positioning of the IOL depend on the degree and gestive of phacodonesis, and small wisps of vitreous are location of zonular disruption. -

44 • M a R C H 2 0
44 • MARCH 2018 Cataract Complications Eight difficult cases that require complex management decisions. HIS PAST NOVEMBER, THE 16TH ANNUAL SPOTLIGHT ON CATARACT SURGERY Symposium at AAO 2017 was entitled “Clinical Decision-Making With Cataract Complications: TYou Make the Call.” Cochaired by Mitchell P. Weikert, MD, and myself, this 4-hour symposium was organized around 8 video cases that presented a range of cataract surgical challenges and complications. The 8 cases were selected from my own practice. As I presented the videos, I would pause at selected points to note a complication or introduce the need to make a management decision. The attendees were then asked to make clinical decisions using their electronic audience response keypads. This was followed by several rapid-fire didactic presentations by invited experts on topics of relevance to the case. Next, a rotating panel of 2 discussants (who had never viewed the case) was asked to make a manage- ment recommendation before the video of the outcome was shown. Following additional audience polling about preferences and practices, the 2 panelists would provide their own opinions and pearls. In all, nearly 40 presenters and panelists spoke about a wide variety of topics, including managing a postvitrectomy cataract, posterior capsular rupture in a multifocal or toric IOL patient, traumatic cataracts, ultrabrunescent cataracts, small pupils, crowded anterior segments, unhappy multifocal IOL patients, iris prolapse, traumatic iris defects, and retained cortex. Alan S. Crandall, MD, concluded the symposium by delivering the 13th annual Academy Charles D. Kelman Lecture, “Phaco at 50: The Collision of Cataract and Glaucoma (Plus).” This EyeNet article reports the results of the 35 audience response questions, accompanied by written commentary from the symposium speakers and panelists. -

IOL Scaffold in Phacoemulsification to Prevent Posterior Capsule Tear
Title: IOL scaffold in phacoemulsification to prevent posterior capsule tear. 1 Dr. Ashraful Huq Ridoy, 2 Dr. Niaz Abdur Rahman 3 Dr. Mahziba Rahman Chowdhury, 4 Dr. Syeed Mehbub Ul Kadir, 5 Dr. Mahbubur Rahman Chowdhury Purpose – To prevent posterior capsule tear and evaluate the post-operative outcomes of phacoemulsification with IOL scaffold technique. Design – Single-center, prospective, interventional, non-comparative, consecutive case series. Materials and Methods – A total of 17 eyes of 17 patients with morgagnian (04 eyes) and hard (nuclear sclerosis grade – IV of 13 eyes) cataract who had undergone phacoemulsification with IOL scaffold technique to prevent posterior capsule tear in a tertiary clinic. All surgeries were performed by a single surgeon. 6.0 mm optic, acrylic, foldable IOL was implanted in all eyes. The pre- operative and post-operative parameters evaluated were uncorrected distance visual acuity, corrected distance visual acuity, cornea status, intraocular pressure and anterior segment inflammation. Results – Posterior capsule tear were prevented in all eyes. At 1-month follow- up, a significant improvement was noted in uncorrected distance visual acuity post-operatively (P-value <0.0001) and 6/6 visual acuity achieved in 71% eyes. Corrected visual acuity 6/6 achieved in 18% eyes. Mean post-operative IOP at 1-day and 1-month follow-up without any medication were 12.79 mmHg and 13.06 mmHg respectively. Immediate post-operative grade-2 anterior segment reaction (02 eyes) and minimal corneal edema (03 eyes) noted which were resolved by 1-month. Conclusion - IOL scaffold provides a safe and effective way to prevent posterior capsule tear in phacoemulsification of morgagnian and hard cataracts, with a good visual outcome. -
Glued IOL Scaffold Technique
TACKLING POSTERIOR CAPSULE RUPTURE AND IOL IMPLANTATION: A VIDEO BASED COURSE MONDAY - 20 th APRIL, 2015: 10.00 AM-11.30 AM, SD CC, ROOM 6 F ; SESSION 20-202 COURSE DESCRIPTION BASIC FUNDAMENTALS Early recognition and management of PC rupture is a key to prevent the sequential complications which can trail and have an adverse effect on the visual output. The following signs should be watched upon for early recognition of a PC rupture. • Sudden deepening of anterior chamber with momentary dilation of pupil • Sudden transitory appearance of red reflex • Difficulty in rotating a previously mobile nucleus • Undue tilting of one pole of nucleus …THE COURSE WILL HIGHLIGHT THE METHODS OF HANDLING POSTERIOR CAPSULE (PC) RUPTURE, ITS EFFECTIVE MANAGEMENT AND VARIOUS MODALITIES OF IOL IMPLANTATION. MANAGEMENT PROTOCOL OF PC BASICS RUPTURE AND IOL IMPLANTATION • In the context of a ruptured posterior capsule, the immediate reflex • Lower down phaco parameters action to be deterred on behalf of the surgeon is to withdraw the • Low flow rate phacoemulsification probe suddenly from the eye. A dispersive OVD from the side port incision should be used to plug-in the posterior • Moderate vacuum capsule defect and fill and stabilise the anterior chamber followed by withdrawal of the phacoemulsification probe. • Low to moderate phaco burst energy to promote • The posterior capsule rupture when small is converted into a posterior capsulorhexis with stable margin facilitating the follow ability implantation of a one piece foldable acrylic IOL in the capsular bag. But if the capsular rupture opening is large, sulcus fixation of an IOL is preferable in cases with adequate anterior capsular margin support. -

IIRSI 2015 Brochure Inside Pages
SCIENTIFIC PROGRAM 3 OCTOBER, 2015 HALL A HALL B HALL C HALL D HALL E HALL F Phaco Panorama-1 Phaco Fundamentals LASIK Lessons IOL Issues Wet Lab (8 am-9:30 am) Cataract-Back 2 the (8 am-10 am) (8 am-10 am) (8 am-10:30 am) (8 am-10 am) Basics (8 am-10:30 am) Phaco Panorama-2 Premium IOL Pointers Women in Ophthalmology Cornea Callisthenics Wet Lab (9:30 am-11 am) (10 am-11:30 am) (10 am-11:30 am) (10:30 am-12:45 pm) (10 am-11:30 am) Cataract-Advancing The Femtorevoluon Techniques &Technology Phaco Fears & Tears Kracking Keratoconus Ophthalmology 20/30- Wet Lab in Cataract Surgery Exploring the Future (10:30 am-1:30 pm) (11:30 am-1:30 pm) (11:30 am-1:30 pm) (11:30 am-1:30 pm) (11 am-1:30 pm) (12:45 pm-1:30 pm) LUNCH BREAK Tips & Tricks Free Papers I: Free Papers II: Cornea & Wet Lab Live Surgery and Inauguraon Cataract & IOL Refracve Surgery (2 pm-6 pm) (2 pm-5 pm) (2 pm-6 pm) (2 pm-6 pm) (2 pm-6 pm) 4 OCTOBER, 2015 HALL A HALL B HALL C HALL D HALL E HALL F The Femto Revoluon in Refracve Surgery-Back 2 the Rena Revisited Phaco Phobia Phakic IOL & (8 am-10 am) Presbyopia Refracve Surgery (8 am-10 am) Basics (8 am-10:30 am) (8 am-10:20 am) (8 am-10 am) New Technology IOLs- Learn from Eye Quest- The Quiz IOL in scky situaons Phaco Fights Cornea Corner the Masters (10 am-12 noon) (10:30 am-12 noon) (10:20 am-12 noon) (10 am-12 noon) (10 am-12 noon) Snags & Soluons OPL (12 noon-1:45 pm) OPL (12 noon-1:45 pm) Mixed Bag (12 noon-2 pm) (12 noon-2 pm) LUNCH BREAK A to Z of Phaco in your Court Maral in Ophthalmology Invenng the Future- Innovaons -

Management of Posterior Capsular Tear
Dr. Harbansh Lal Hony. Treasurer AIOS Co-Chairman, Dept. of Ophthalmology, Sir Ganga Ram Hospital, Delhi Director, Delhi Eye Centre Email: [email protected] Mob: 9810239206 All India Ophthalmological Society Office Bearers PRESIDENT Dr. Anita Panda PRESIDENT ELECT Dr. Quresh B. Maskati VICE PRESIDENT Dr. Debasish Bhattacharya HONY. GENERAL SECRETARY Dr. Lalit Verma JOINT SECRETARY Dr. Sambasiva Rao V. HONY. TREASURER Dr. Harbansh Lal JOINT TREASURER Dr. Ruchi Goel EDITOR - JOURNAL Dr. S. Natarajan EDITOR PROCEEDINGS Dr. Samar Kumar Basak CHAIRMAN SCIENTIFIC COMMITTEE Dr. D. Ramamurthy CHAIRMAN - ARC Dr. Ajit Babu Majji IMMEDIATE PAST PRESIDENT Dr. N.S.D. Raju Posterior capsular tear or PCT is one of the disastrous complications of cataract surgery. Although common in the learning stages, it can occur even in the hands of experienced surgeons – specially if they are in hurry or overconfident. The situation does create panic in the mind of operating surgeon – the surgeon sometimes tends to do lot of unwarranted steps. If not managed properly, the outcome can be disastrous for the eye; however, if managed properly the results can be quite rewarding. This booklet by Dr. Harbansh Lal, one of the pioneers of Cataract Surgery, describes in a very lucid and practical way the do’s & don’ts of PCR. Complications do happen in the best of hands but the true competence of a surgeon is judged by how he handles complications. I am sure this masterpiece by Dr Harbansh Lal will help everyone. Dr. Anita Panda President, AIOS All India Ophthalmological Society (Academic & Research Committee) Chairman Dr. Ajit Babu Majji Medical Director, Centre for Sight Ashoka Capitol Building, Road # 2, Banjara Hills, Hyderabad - 500 034 Ph: 040-40045500 Mobile: 09391026292 E-mail: [email protected] Members Dr. -

Prevention, Management of Subluxated Crystalline Lenses and Iols Glued Endocapsular Ring, Glued IOL, and IOL Scaffold Techniques May Address These Issues
CATARACT SURGERY BONUS FEATURE Prevention, Management of Subluxated Crystalline Lenses and IOLs Glued endocapsular ring, glued IOL, and IOL scaffold techniques may address these issues. BY SOOSAN JACOB, MS, FRCS, DNB; AND AMAR AGARWAL, MS, FRCS, FRCOPHTH n cataract surgery, avoiding subluxation or enlarge- frank dialysis has not yet occurred. The endocapsular ment of an existing subluxation of the crystalline lens is ring expands and stabilizes the capsular bag during of prime importance. Similarly, intra- or postoperative cataract surgery. It creates centrifugal forces that result subluxation of an IOL is a complication that all sur- in redistribution from stronger to weaker areas. The Igeons wish to avoid. Basic principles in pre- and intraop- endocapsular ring also makes the capsule taut and gives erative planning can help greatly to minimize the risk of counter-traction to all traction maneuvers. The surgeon these events and to manage them if they occur. may opt to place capsular hooks initially to stabilize Preoperatively, factors that can predispose a patient the bag and then implant the endocapsular ring after to intraoperative subluxation should be evaluated and cortex aspiration. dealt with appropriately. These include conditions that If, despite these precautions, subluxation does occur, convey zonular weakness, such as pseudoexfoliation, it should be managed according to the degree of zonular trauma, Marfan syndrome, ectopia lentis, hypermature dialysis. Smaller subluxations (up to 3 or 4 clock hours) cataract, high myopia, megalophthalmos, postvitrectomy can be managed by inserting an endocapsular ring. Larger status, and any other cause of preoperative phacodonesis. subluxations require scleral anchoring of the capsular bag Additionally, any cataract that is expected to entail difficult and its contents; we prefer to do this through the use of and complicated surgery can lead to an increased risk of a glued endocapsular ring. -

1-10-14 CSEP Program.Pdf
Eye M.D. Education Mission Statement: We are committed to advancing the highest standards of eye care through continuing education activities. The CSEP Semi-annual Scientific Education Programs arededicated to improving and protecting our patient’s vision and eye health by presenting advances in the diagnosis and treatment of eye disease. Our target audience includes ophthalmologists and their staff, including office managers and technicians. Activities range from didactic lectures to participatory activities, and whenever possible are approved for CME credit. We expect that our audience will incorporate best practices, as presented, into their daily practice. Specific competency, performance and patient outcome goals that will result from the program will be proposed by the presenters and evaluated by the participants. The CSEP Annual Scientific Education Programs are an opportunity for ophthalmologists to identify and discuss critical issues facing their profession. These programs are designed to present recent advances in the diagnosis and treatment of eye disease, offer - ing symposia, scientific papers and videos. The CSEP programs are designed to meet the clinical and ed - ucational needs of its members and the objectives set forth by the CSEP education committee. Vincent deLuise, M.D. CSEP Education Chair SPECIAL ACKNOWLEDGMENT AND THANKS to our Sponsors TITANIUM Genentech Regeneron PLATINUM Alcon Laboratories Allergan GOLD McLeod Optical Company Precision Optical Company SILVER Carl Zeiss Medical Connecticut State Library for the Blind & Physically Handicapped Diopsys, Inc. Eaglevision and Rhein Medical Eye Formatics SILVER (cont’d) EyeMD EMR Healthcare Systems, Inc. Fallon Wellness Pharmacy Glaukos Heidelberg Engineering Hoya Vision Care ifa united i-tech, Inc. IOP Ophthalmic0 Lion Low Vision Center Marco MEDENT Mobius Therapeutics, LLC MST (Micro Surgical Technology) New World Medical, Inc. -

Subjective Quality of Vision
LETTERS TO THE EDITOR Subjective Quality of Vision Colm McAlinden, PhD Jyoti Khadka, PhD To the Editor: Konrad Pesudovs, PhD We read with interest the study by Sia et al,1 which Bedford Park, South Australia appeared in the January 2012 issue of the Journal of Eirini Skiadaresi, MD Refractive Surgery, comparing epi-LASIK and photore- Swansea, United Kingdom fractive keratectomy, particularly with regards to the questionnaire used to assess quality of vision. The The authors have no proprietary interest in the materials presented questionnaire consisted of a 10-point scale ranging herein. from 1 (no symptoms) to 10 (severe, disabling symp- toms). The results found the subjective optical quality REFERENCES was the same between the two procedures for vision 1. Sia RK, Coe CD, Edwards JD, Ryan DS, Bower KS. Visual out- fl uctuations, double vision, glare, light sensitivity, comes after epi-LASIK and PRK for low and moderate myopia. J Refract Surg. 2012;28(1):65-71. halos, starbursts, patient satisfaction, postoperative 2. Thomee R, Grimby G, Wright BD, Linacre JM. Rasch analysis of vision quality, and the chance to have the procedure Visual Analog Scale measurements before and after treatment again. of Patellofemoral Pain Syndrome in women. Scand J Rehabil Med. We would like to propose that a potential reason 1995;27(3):145-151. why no difference was found between the two proce- 3. Pesudovs K, Noble BA. Improving subjective scaling of pain dures is the quality of the questionnaire, which has a using Rasch analysis. J Pain. 2005;6(9):630-636. number of fl aws. -

Cataract Surgical Complications
Cataract Surgical Complications Eighteen cases cover the full spectrum of surgical complications, from the common to the rare—and from the spectacular save to the demoralizing outcome. HIS PAST OCTOBER, THE 17TH ANNUAL SPOTLIGHT ON CATARACT SURGERY SYM- posium at AAO 2018 was entitled “Pressure Cooker: Managing Nerve-racking Complications.” TCochaired by Mitchell P. Weikert, MD, and myself, this four-hour case-based video symposium focused on cataract and IOL surgical complications. Even the very best cataract surgeons suffer complications that challenge us to react, think, and operate under pressure. How and what we learn from our mistakes makes us better ophthalmologists. For this symposium, 18 cataract experts presented stressful cases in which something went wrong, with complications that tested their skills, decision-making, and nerves. What did they learn, and what would they do differently? At critical decision points during the case, the video was paused, and the attendees were asked to make clinical decisions using electronic audience response pads. Next, two discussants (who had never viewed the case) were asked to make their own management recommendations and to comment on the audience responses before the video of the outcome was shown. The audience voted for the best teaching cases and for those surgeons who displayed the most courage, both in the OR and at the podium. Complications included anterior capsule tears (with and without posterior capsular extension), implantation of the wrong IOL, intraocular bleeding, haptic misadventures and subluxated IOLs, iris prolapse and iatrogenic iridodialysis, aqueous misdirection, suprachoroidal hemorrhage, descending nuclei and IOLs, IOL exchange complications, and capsules or zonules torn at virtually every stage of surgery. -

Dr Ashvin Agarwal
DR ASHVIN AGARWAL WORK TITLE Executive Director Chief Clinical Officer & Chairman of Clinical Board @ Dr. Agarwal’s Eye Hospital INTERNATIONAL COMMITTEE POSITIONS EyeConnect International – Co founder ISRS Webinar Task Force Chair 222, TTK Road, Alwarpet, ISRS Cataract Refractive committee member Chennai – 600018, India AAO ONE Network Member PROFILE +91 94450 21736 After completing his med school and post-graduation in ophthalmology, Ashvin gave his International Council of [email protected] Ophthalmology - ICO Part 1. He then worked with in Bascom Palmer Institute, Miami, Florida & Price Vision Group, Indianapolis. Trained in Refractive and Corneal Surgeries. Headed back to Dr Agarwal eye hospital Chennai, India. Worked in cataract division and had been www.drashvinagarwal.com working ever Since then he has been practicing at Dr. Agarwal’s Eye Hospital till date and is the Managing Director of Orbit Healthcare Pvt Ltd. Dr. Ashvin has performed over 15000 surgeries so far. He EDUCATIONAL QUALIFICATIONS specializes in a niche segment of complex Cataract care M.B.,B.S. - (April 2007) management, Corneal refractive surgeries and anterior segment R.Ramaiah Medical College, Bangalore repair procedures. M.S. Ophthalmology - (Apr 2010) He is the Chief clinical officer of Dr. Agarwal’s eye hospitals group Rajah Muthiah Medical College, which have over 95 locations globally, he takes strategic & Raja Annamalai Nagar, Chidambaram administrative decisions towards sustenance and clinical quality across the group. ORGANIZING EVENTS World Webinar on Cataract and Refractive Surgery – Co Founder IIRSI – Organizer since 2011 Rising Stars in Ophthalmology – Co Founder HOBBIES RETICON - Programme director since 2014 Reading, Swimming, Squash, Dr. Agarwal’s Grand Rounds – Organizer, Monthly Since 2018 Cricket, Tennis & Watching Movies.