The Embryonic Origins of Left-Right Asymmetry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopic Cholecystectomy in Situs Inversus Totalis: Case Report with Review of Techniques
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 59 (2019) 208–212 Contents lists available at ScienceDirect International Journal of Surgery Case Reports j ournal homepage: www.casereports.com Laparoscopic cholecystectomy in situs inversus totalis: Case report with review of techniques ∗ Omar AlKhlaiwy, Ahmed Mohammed AlMuhsin , Eman Zakarneh, Mohamed Yassin Taha Department of General Surgery, King Fahd Military Medical Complex, Dhahran, Saudi Arabia a r t i c l e i n f o a b s t r a c t Article history: INTRODUCTION: Situs inversus totalis (SIT) is a congenital disorder in which the visceral organs are mir- Received 18 January 2019 rored from their normal anatomical position. Diagnosis and management of cholelithiasis in patient with Received in revised form 21 March 2019 SIT poses a challenge due to the underlying anatomical variation. Accepted 23 May 2019 PRESENTATION OF CASE: We report a case of a 40-year-old male patient who presented with an intermit- Available online 31 May 2019 tent history of epigastric and left upper quadrant pain for one month. Clinical assessment and radiological investigations confirmed the presence of cholelithiasis with evidence of SIT. The patient underwent elec- Keywords: tive laparoscopic cholecystectomy with no complication and he had an uneventful recovery. Various Situs inversus totalis intraoperative modification has been made to overcome the technical difficulties encountered due to the Laparoscopic cholecystectomy underlying anatomical variation. Case report DISCUSSION: Since the first successful laparoscopic cholecystectomy in patient with SIT performed in 1991, 85 cases have been reporsted in the literature. Surgeons managed to overcome the technical dif- ficulties by adopting various modification in the techniques compared to the conventional laparoscopic cholecystectomy. -

Isolated Levocardia with Situs Inversus Without Cardiac Abnormality in Fetus: Prenatal Diagnosis and Management
A L J O A T U N R I N R A E L P Case Report P L E R A Perinatal Journal 2020;28(1):48–51 I N N R A U T A L J O ©2020 Perinatal Medicine Foundation Isolated levocardia with situs inversus without cardiac abnormality in fetus: prenatal diagnosis and management Mucize Eriç ÖzdemirİD , Oya Demirci İD Department of Perinatology, Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital., Health Sciences University, Istanbul, Turkey Abstract Özet: Kardiyak anomalisi olmayan fetüste situs inversuslu izole levokardi: Prenatal tan› ve yönetim Objective: Isolated levocardia is a situs abnormality that the heart is Amaç: ‹zole levokardi, kalbin normal levo pozisyonunda oldu¤u in the normal levo position, but the abdominal viscera are in dextro fakat abdominal iç organlar›n dekstro pozisyonunda oldu¤u bir si- position. Most cases are accompanied by structural heart anomalies. tus anomalisidir. Ço¤u olguda yap›sal kalp anomalileri de efllik et- In this case, we aimed to present a fetus with isolated levocardia with- mektedir. Çal›flmam›zda, kardiyak anomalisi olmayan izole levo- out cardiac abnormality. kardili bir fetüsü sunmay› amaçlad›k. Case: The mother was referred to our clinic with a suspicion of fetal Olgu: Olgumuz, 22. gebelik haftas›nda fetal dekstrokardi flüphe- dextrocardia at 22 weeks of gestation. When detailed examination was siyle klini¤imize sevk edildi. Planlanan detayl› ultrason muayene- planned by ultrasonography isolated levocardia was detected in fetus. sinde, fetüste izole levokardi tespit edildi. Fetal ekokardiyografide There were no cardiac abnormalities in fetal echocardiography. Fetus hiçbir kardiyak anomali görülmedi. -

Coronary Angiography in a Patient with Situs Inversus and Dextrocardia
Case Reports Olgu Sunumlar› 455 Coronary angiography in a patient with situs inversus and dextrocardia Situs inversuslu¸ ve dekstrokardili bir hastada koroner anjiyografi Mehmet Çilingiro¤lu, Mohammad-Abdul Waheed, Nuri Akkufl Department of Interventional Cardiology, Faculty of Medicine, University of Cincinnati, Cincinnati, Ohio, USA Introduction Dextrocardia occurs rarely, with a frequency estimated at 1:10,000 (1). There is scant information available to the angiographer faced with performing catheterization in a patient with dextrocardia. We report successful coronary angiography in a patient with dextrocardia associated with situs inversus totalis. Catheterization in our patient was performed without difficulty using standard techniques. Case Report A 50-year old white male with known dextrocardia presented with severe substernal chest pains. His symptoms were reproducible with mild to moderate exertion. Physical examination was unremarkable except for findings consistent with dextrocardia. Chest X ray was remarkable for dextrocardia and right-sided stomach bubble. Electrocardiogram, with the properly reversed leads for dextrocardia, showed left ventricular hypertrophy. Two-dimensional echocardiogram Figure 1. Left ventricular angiogram taken in LAO view showed a left-sided liver and dextrocardia without other abnormalities. LAO-left anterior oblique Exercise testing using modified Bruce protocol reproduced his symptoms within the first stage of the test. Cardiac catheterization was performed from the right femoral artery. Catheters were passed using mirror-image angiographic angles. A 6-French angulated pigtail catheter was passed into left ventricle and a left ventriculogram was obtained using 30-degree left anterior oblique imaging (Fig. 1). Selective coronary angiogram was performed using left and right 6-French 4 cm Judkins diagnostic catheters (400 right anterior oblique) (Fig. -

The Genetics of Laterality Defects
Laterality Disturbance and Heart Defects The information which is used to plan our bodies is carried by our genes, half of which we inherit from our mother and half from our father. This hereditary information can carry errors which can ultimately lead to errors in the way parts of our body, such as the heart, develop. What does laterality disturbance mean? We should not be symmetrical Although our bodies are mostly symmetrical, several of our internal organs are not: for example, the heart is on the centre-left of the chest, one side pumps blood to the lungs and the other to the body, and the stomach is on the left, the liver is on the right, and the spleen on the left. Very early in pregnancy - about the fourth or fifth week - the cells which make our various organs, receive signals which program them to produce structures for the left or right side of the body. If the cells are programmed incorrectly the body’s left-right plan or ‘laterality’ is disturbed. Mirror image About one of every ten thousand people has a mirror image arrangement, with the heart, spleen and stomach on the right and the liver on the left side. This is called situs inversus and results in normal relationships between the left-right positions of the organs - for example the arteries carrying red blood from the heart will travel towards and reach the correct organ. Symmetrical Occasionally some or all of the organs are symmetrical - this is called ‘heterotaxy’. The most extreme form of heterotaxy is ‘isomerism’ (pronounced eyes-om-er-ism) where two right sides or two left sides develop. -

Imaging Evaluation of the Heterotaxy Syndrome
Gastroenterology & Hepatology: Open Access Review Article Open Access Imaging evaluation of the heterotaxy syndrome Abstract Volume 10 Issue 2 - 2019 Situs ambiguus, or heterotaxy, is a rare syndrome characterized by an abnormal José Maximiliano Garófano-Jerez,1,2,3 Elena arrangement of the internal organs in the chest and abdomen and is usually associated Benedicto-Hernández,2 Juan de Dios with congenital heart diseases. Despite its variable presentation, heterotaxy syndrome 4 can be classified into heterotaxy with polysplenia or left isomerism, and heterotaxy López-González Gila, Juan de Dios López- 1,2 with asplenia or right isomerism. The different imaging techniques reveal the González Garrido characteristic imaging findings that allow an accurate description of each patient’s 1Departamento de Radiología y Medicina Física, Spain 2 specific anatomy. Evaluation of the position of the following structures relative to Facultad de Medicina, Universidad de Granada, Spain 3 midline is crucial: atria, cardiac apex, venous drainage, aorta, stomach, liver and Servicio de Radiodiagnóstico, Hospital Universitario San Cecilio de Granada, Spain gallbladder, as well as the presence or absence of intestinal malrotation, number of 4Servicio de Nefrología del Hospital Universitario Virgen de las spleens, and presence of tri- or bilobed lungs. Nieves de Granada, Spain We present a case report of a 42-year-old man with dextrocardia underwent laparoscopic cholecystectomy for cholelithiasis. Subsequently, a complete resection Correspondence: Juan de Dios López-González Garrido, of the common bile duct required reconstruction of the biliary tract. During six years Profesor Titular de Universidad. Departamento de Radiología y Medicina Física de la Facultad de Medicina. Avda. -
Laparoscopic Cholecystectomy in a Patient with Situs Inversus Totalis
Bangladesh Crit Care J March 2021; 9 (1): 43-45 Case Report Laparoscopic Cholecystectomy in a patient with Situs Inversus Totalis Samail Shahjahan1, Anisur Rahman*2 DOI: https://doi.org/10.3329/bccj.v9i1.53057 Abstract: There are diagnostic and therapeutic challenges in cases of symptomatic gall bladder disease in patients with situs inversus totalis (SIT), where there is complete reversal of visceral topography in thorax, abdomen or both. The difficulty to treat these patients with conventional laparoscopic cholecystectomy may be more pronounced for right handed surgeon and requires modifications in working ports and their positions. We present a case of laparoscopic cholecystectomy in a patient with SIT, and describe the technical details that enable the safe conclusion of the operation. Keywords: Laparoscopic Cholecystectomy, Situs Inversus totalis(SIT) Background vomiting, and denied having fever or jaundice. A diagnostic Situs inversus is a rare autosomal recessive disorder in which workup included abdominal ultrasonogrphy, revealed left there is opposite (mirror image) orientation of thoracic or sided contracted gall bladder containing multiple stones. It abdominal organs (partial) or both (total). Incidence of such also showed the liver is on left side and the spleen on right condition occurs to be in 1 in 5000 to 20000 live births1.This side of abdomen. So a diagnosis of situs inversus with condition can also associated with a number of conditions, cholelithiasis was made. Lab results ruled out infection like Kartagener’s syndrome (bronchiectasis, sinusitis, situs (WBC=13000/cumm, Neutrophil 63% and Lymphocyte inversus) and cardiac anomalies, although infrequently2. 31.5%) or liver function abnormalities. Pre-operative chest X-ray P/A view and 2D Echocardiogram revealed mirror Laparoscopic cholecystectomy in such patients is challenging image dextrocardia (Figure 1). -

White Matter Asymmetries in Human Situs Inversus Totalis
Brain Structure and Function (2019) 224:2559–2565 https://doi.org/10.1007/s00429-019-01904-x SHORT COMMUNICATION White matter asymmetries in human situs inversus totalis Lieselot Mannaert1 · Helena Verhelst1,2 · Robin Gerrits1,2 · Stephanie Bogaert2,3 · Guy Vingerhoets1,2 Received: 18 January 2019 / Accepted: 6 June 2019 / Published online: 13 June 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Difusion weighted imaging (DWI) was used to investigate white matter asymmetries in participants with situs inversus totalis (SIT) and matched controls. Regardless of visceral condition, hemispheric diferences were found for the arcuate fasciculus (ARC) and the superior longitudinal fasciculus (SLF), which are involved in language and visuospatial function- ing, respectively. The ARC appears lateralized to the left hemisphere, analogous to the left lateralization of functional areas associated with language. The SLF, on the other hand, is lateralized to the right, corresponding with rightward lateralization of visuospatial functioning. Interestingly, SIT participants show a signifcantly lower number of streamlines in the Uncinate Fasciculus (UNC). In addition, UNC volume appears associated with measures of cognitive performance, a fnding in line with previously reported performance diferences between SIT participants and controls. Keywords Situs inversus totalis · White matter asymmetry · Lateralization · Arcuate fasciculus · Uncinate fasciculus · Superior longitudinal fasciculus Introduction Recently, we compared brain structural and functional asymmetries in 15 participants with SIT and 15 matched Situs inversus totalis (SIT) is a rare anatomical condition in control participants. We aimed to investigate whether which the internal organs are in mirrored position compared humans show an association between visceral and brain to their typical location with the heart on the left and the asymmetries, an observation reported in other animal spe- liver on the right (Grimes and Burdine 2017). -

Left-Right Asymmetry in Vertebrate Embryogenesis
Michael Levin Summary Embryonic development results in animals whose body plans exhibit a variety of symmetry types. While significant progress has been made in understanding the molecular events underlying the early specification of the antero-posterior and dorso-ventral axes, little information has been available regarding the basis for left-right (LR) differences in animal morphogenesis. Recently however, important advances have been made in uncovering the molecular mechanisms responsible for LR patterning. A number of genes (including well-known signaling molecules such as Sonic hedgehog and activin) are asymmetrically expressed in early chick embryos, well before the appearance of morphological asymmetries. One of these, nodal, is asymmetrically expressed in frogs and mice as well, and its expression is altered in mouse mutants exhibiting defects in laterality. In the chick, these genes regulate each other in a sequential cascade, which Accepted independently determines the situs of the heart and other organs. 20 December 1996 Introduction ferences between the left and right sides of an animal’s mor- The geometrical invariance known as symmetry is a striking phology. This specifically excludes pseudo-random charac- feature of developmental morphology during embryogen- teristics such as coat colors, and minor stochastic deviations esis. There are several types, such as translational symme- due to developmental noise. Thus, to count as true LR asym- try (resulting in repeated units, such as embryonic somites or metry, a feature has to appear consistently in all normal indi- millipede segments) and reflectional symmetry (resulting viduals. Here, I also purposefully neglect behavioral/sensory from two or more sections of an organism looking the same asymmetries (such as lobster claws, which are determined to some level of detail on either side of a symmetry line). -
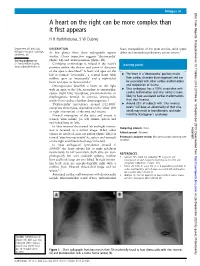
A Heart on the Right Can Be More Complex Than It First Appears
Images in... BMJ Case Reports: first published as 10.1136/bcr-2013-201046 on 19 September 2013. Downloaded from A heart on the right can be more complex than it first appears H R Haththotuwa, S W Dubrey Department of Cardiology, DESCRIPTION heart, transposition of the great arteries, atrial septal Hillingdon Hospital, Uxbridge, At first glance these chest radiographs appear defect and anomalous pulmonary venous return.3 Middlesex, UK similar. Closer inspection suggests ‘dextrocardia’ Correspondence to (figure 1A) and ‘dextroposition’ (figure 1B). ’ Dr Simon William Dubrey, Confusing terminology is helped if the heart s Learning points [email protected] position within the thorax and point of direction of the apex is described.1 A heart and apex on the ▸ ‘ ’ left is termed ‘levocardia’, a central heart with The heart in a dextrocardia position results midline apex as ‘mesocardia’ and a right-sided from cardiac chamber disarrangement and can heart and apex as ‘dextrocardia’. be associated with other cardiac malformations Dextroposition describes a heart on the right and malposition of viscera. ▸ with an apex to the left, secondary to extracardiac Situs ambiguous has a 100% association with causes (right lung hypoplasia, pneumonectomy or cardiac malformation and situs solitus is more diaphragmatic hernia). In contrast, dextrocardia likely to have associated cardiac malformations results from cardiac chamber disarrangement.2 than situs inversus. 2 ▸ ‘ ‘Dextrocardia’ (prevalence around 1/12 000) Around 20% of subjects with situs inversus ’ comprises three types, depending on the ‘situs’ (left totalis will have an abnormality of their cilia, or right orientation) of the atria and viscera. which may result in bronchiectasis and male ’ Normal orientation of the atria and viscera is infertility (Kartagener s syndrome). -

Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis
Edinburgh Research Explorer Brain structural and functional asymmetry in human situs inversus totalis Citation for published version: Vingerhoets, G, Li, X, Hou, L, Bogaert, S, Verhelst, H, Gerrits, R, Siugzdaite, R & Roberts, N 2018, 'Brain structural and functional asymmetry in human situs inversus totalis' Brain Structure and Function. DOI: 10.1007/s00429-017-1598-5 Digital Object Identifier (DOI): 10.1007/s00429-017-1598-5 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Brain Structure and Function General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 24. Jul. 2019 Brain Structure and Function Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis --Manuscript Draft-- Manuscript Number: BSAF-D-17-00474R1 Full Title: Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis Article Type: Original Article Keywords: brain -

Case Reports of Situs Inversus Totalis and Dextrocardia in Sprague Dawley Rats
veterinary sciences Case Report Case Reports of Situs Inversus Totalis and Dextrocardia in Sprague Dawley Rats Reynaldo Oliva Hernández 1, Jordan D. Lewicky 2, Nya L. Fraleigh 2 and Hoang-Thanh Le 2,3,4,* 1 Finlay Institute of Vaccine Research and Production, Havana 11600, Cuba 2 Health Sciences North Research Institute (HSNRI), Sudbury, ON P3E2H3, Canada 3 Northern Ontario School of Medicine (NOSM), Sudbury, ON P3E2C6, Canada 4 Chemistry & Biochemistry and Biology Departments, Laurentian University, Sudbury, ON P3E2C6, Canada * Correspondence: [email protected] Received: 28 June 2019; Accepted: 9 August 2019; Published: 15 August 2019 Abstract: Situs inversus totalis is a condition where there is a transposition of all internal organs from their normal anatomical location. This infrequent and rare congenital condition has been described in several species of mammals. Dextorcardia is a series of conditions associated with an abnormal congenital positioning of the heart, and is often associated with situs inversus totalis. Here we report a case of situs inversus totalis and two cases of dextrocardia identified in Sprague Dawley rats during gross necropsy evaluations at both the Health Sciences North Research Institute (HSNRI) in Canada and Finlay Institute of Vaccine Research and Production in Cuba. The intent of this report is to share our findings and aid in the accumulation of data on these rare conditions. Keywords: Situs inversus totalis; dextrocardia; Sprague Dawley rats 1. Introduction Situs inversus totalis (SIT) is a rare congenital malformation characterized by the complete reversal (transposition) of the normal location of the thoracic and abdominal organs [1]. SIT is a very rare condition, with the incidence rate in humans estimated to be 0.01–0.02% [2], within which there is an equal prevalence among males and females [3]. -

Visceral Situs, Heart Position, and Aortic Arch Position 69
Visceral Situs, Heart Position, 7 and Aortic Arch Position The second step in interpretation of a chest radiograph and (6) the azygos vein. The basic concepts regarding vis- obtained in a new patient is to ascertain visceral situs, the ceral situs is discussed in detail in Chapter 2. heart position, and the position of the aortic arch relative In situs solitus, the gastric air bubble is on the left side, to the trachea. For example, the radiographic report may and the larger lobe of the liver is on the right side (Fig. 7.2). start with this sentence: “The radiograph shows situs solitus, The splenic tip can often be identified when the stomach levocardia and a left aortic arch.” and adjacent bowel loops are filled with air. When the bronchial air column can be traced, an asymmetric bronchial branching pattern with a short right and a long left main bronchus can be appreciated on the frontal radi- ■ Visceral Situs ograph. Normally the left main bronchus is 1.5 to 2 times longer than the right main bronchus. At the pulmonary Visceral situs refers to the pattern of arrangement of the hilum, the left pulmonary artery is seen slightly higher body organs relative to the midline. There are four types than the right pulmonary artery. The left pulmonary of visceral situs: situs solitus, situs inversus, heterotaxy artery is seen above the left upper lobe bronchus, with thoracic right isomerism, and heterotaxy with whereas the right pulmonary artery (in fact, its descend- thoracic left isomerism (Fig. 7.1).1–4 Visceral heterotaxy ing branch) is seen below the right upper lobe bronchus.