OFFICIAL RECORD of PROCEEDINGS Wednesday, 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UC San Diego UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title Powerful patriots : nationalism, diplomacy, and the strategic logic of anti-foreign protest Permalink https://escholarship.org/uc/item/9z19141j Author Weiss, Jessica Chen Publication Date 2008 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO Powerful Patriots: Nationalism, Diplomacy, and the Strategic Logic of Anti-Foreign Protest A Dissertation submitted in partial satisfaction of the Requirements for the degree Doctor of Philosophy in Political Science by Jessica Chen Weiss Committee in charge: Professor David Lake, Co-Chair Professor Susan Shirk, Co-Chair Professor Lawrence Broz Professor Richard Madsen Professor Branislav Slantchev 2008 Copyright Jessica Chen Weiss, 2008 All rights reserved. Signature Page The Dissertation of Jessica Chen Weiss is approved, and it is acceptable in quality and form for publication on microfilm and electronically: Co-Chair Co-Chair University of California, San Diego 2008 iii Dedication To my parents iv Epigraph In regard to China-Japan relations, reactions among youths, especially students, are strong. If difficult problems were to appear still further, it will become impossible to explain them to the people. It will become impossible to control them. I want you to understand this position which we are in. Deng Xiaoping, speaking at a meeting with high-level Japanese officials, including Ministers of Foreign Affairs, Finance, Agriculture, and Forestry, June 28, 19871 During times of crisis, Arab governments demonstrated their own conception of public opinion as a street that needed to be contained. Some even complained about the absence of demonstrators at times when they hoped to persuade the United States to ease its demands for public endorsements of its policies. -

Grand Bauhinia Medal (GBM)
Appendix Grand Bauhinia Medal (GBM) The Honourable Chief Justice CHEUNG Kui-nung, Andrew Chief Justice CHEUNG is awarded GBM in recognition of his dedicated and distinguished public service to the Judiciary and the Hong Kong community, as well as his tremendous contribution to upholding the rule of law. With his outstanding ability, leadership and experience in the operation of the judicial system, he has made significant contribution to leading the Judiciary to move with the times, adjudicating cases in accordance with the law, safeguarding the interests of the Hong Kong community, and maintaining efficient operation of courts and tribunals at all levels. He has also made exemplary efforts in commanding public confidence in the judicial system of Hong Kong. The Honourable CHENG Yeuk-wah, Teresa, GBS, SC, JP Ms CHENG is awarded GBM in recognition of her dedicated and distinguished public service to the Government and the Hong Kong community, particularly in her capacity as the Secretary for Justice since 2018. With her outstanding ability and strong commitment to Hong Kong’s legal profession, Ms CHENG has led the Department of Justice in performing its various functions and provided comprehensive legal advice to the Chief Executive and the Government. She has also made significant contribution to upholding the rule of law, ensuring a fair and effective administration of justice and protecting public interest, as well as promoting the development of Hong Kong as a centre of arbitration services worldwide and consolidating Hong Kong's status as an international legal hub for dispute resolution services. The Honourable CHOW Chung-kong, GBS, JP Over the years, Mr CHOW has served the community with a distinguished record of public service. -

Standoff at Tiananmen: Recollections of 1989: the Making of Goddess of Democracy
2019/4/23 Standoff At Tiananmen: Recollections of 1989: The Making of Goddess of Democracy 更多 创建博客 登录 Standoff At Tiananmen How Chinese Students Shocked the World with a Magnificent Movement for Democracy and Liberty that Ended in the Tragic Tiananmen Massacre in 1989. Relive the history with this blog and my book, "Standoff at Tiananmen", a narrative history of the movement. Home Days People Documents Pictures Books Recollections Memorials Monday, May 30, 2011 "Standoff at Tiananmen" English Language Edition Recollections of 1989: The Making of Goddess of Democracy Click on the image to buy at Amazon "Standoff at Tiananmen" Chinese Language Edition On May 30, 1989, the statue Goddess of Democracy was erected at Tiananmen Square and became one of the lasting symbols of the 1989 student movement. The following is a re-telling of the making of that statue, originally published in the book Children of Dragon, by a sculptor named Cao Xinyuan: Nothing excites a sculptor as much as seeing a work of her own creation take shape. But although I was watching the creation of a sculpture that I had had no part in making, I nevertheless felt the same excitement. It was the "Goddess of Democracy" statue that stood for five days in Tiananmen Square. Until last year I was a graduate student at the Central Academy of Fine Arts in Beijing, where the sculpture was made. I was living there when these events took place. 点击图像去Amazon购买 Students and faculty of the Central Academy of Fine Arts, which is located only a short distance from Tiananmen Square, had from the beginning been actively involved in the demonstrations. -

Zhou Fengsuo Looks Back at Humanitarian China's Journey in 2019 in an Interview (Full Transcript)
Video link: https://youtu.be/tmaJzU0Fyxw Zhou Fengsuo looks back at Humanitarian China's journey in 2019 in an interview (full transcript) Host: This is the Chen Pokong Fengyun Show and I am special correspondent Wu Yun. Today, we’ve invited Mr Zhou Fengsuo, former student leader during the Tiananmen Square protests of 1989 and president of Humanitarian China. Welcome, Mr Zhou. We would like you to tell us about Humanitarian China’s work during 2019 and its future plans . Zhou Fengsuo: Thank you. It’s a privilege for me to have the opportunity to talk about our work during the past year and our future plans. During the past year, human rights in China continued to deteriorate, which brought us huge challenges. The need is great. The most important work Humanitarian China does is to assist prisoners of conscience in China; we provide humanitarian relief to over 100 people annually. This group now consists of a wide range of people, including groups we have been supporting such as human rights lawyers, former Democracy Party members and Tiananmen Square Massacre victims, and some new groups that have emerged this year, for instance, the Hong Kong protesters and those arrested over rights issues that had previously been regarded as relatively minor in China, for instance, labor rights, woman rights and equality. The CCP is becoming increasingly paranoid and treating everyone as an enemy, which results in many different groups being targeted. We do our best to provide humanitarian relief. Our most crucial task is to understand their situation and needs, and to promote international awareness of human rights violations. -

Hong Kong Country Overview
HONG KONG COUNTRY OVERVIEW INTRODUCTION Electric, eclectic, energizing, nonstop, traditional, cosmopolitan, international; there are so many words to describe Hong Kong, one simply has to visit to experience it all. Hong Kong was a British colony from the mid-19th century until 1997 when China resumed sovereignty. The city now operates as a Special Administrative Region (SAR) under China’s ‘one country – two rule system.’ A haven for consumerists, Hong Kong offers some of the best shopping anywhere in the world. The infrastructure is modern and developed which makes getting around easy. On top of that, because of the city’s long history with the western world, English is spoken everywhere making Hong Kong a relatively easy destination to visit compared to other parts of China. 2 ABOUT HONG KONG LANDSCAPE Hong Kong is located at the delta of the Pearl River on China’s Southeast coast. The city is made up of Hong Kong island, and several areas on the mainland peninsula known as Kowloon and the New Territories. In total, the land area is over 1100 km2. CLIMATE Hong Kong enjoys a humid subtropical climate with hot, humid summers and relatively mild winters. It is most likely to rain during the summer months (June, July, August) and this is, therefore, the low travel season. The most popular seasons to visit are Spring with mild temperatures and only occasional rain and autumn which is usually sunny and dry. PEOPLE There are approximately 7.3 million people living in Hong Kong, 95% of whom are of Chinese descent (Mainly Canton people). -

List of Recognized Villages Under the New Territories Small House Policy
LIST OF RECOGNIZED VILLAGES UNDER THE NEW TERRITORIES SMALL HOUSE POLICY Islands North Sai Kung Sha Tin Tuen Mun Tai Po Tsuen Wan Kwai Tsing Yuen Long Village Improvement Section Lands Department September 2009 Edition 1 RECOGNIZED VILLAGES IN ISLANDS DISTRICT Village Name District 1 KO LONG LAMMA NORTH 2 LO TIK WAN LAMMA NORTH 3 PAK KOK KAU TSUEN LAMMA NORTH 4 PAK KOK SAN TSUEN LAMMA NORTH 5 SHA PO LAMMA NORTH 6 TAI PENG LAMMA NORTH 7 TAI WAN KAU TSUEN LAMMA NORTH 8 TAI WAN SAN TSUEN LAMMA NORTH 9 TAI YUEN LAMMA NORTH 10 WANG LONG LAMMA NORTH 11 YUNG SHUE LONG LAMMA NORTH 12 YUNG SHUE WAN LAMMA NORTH 13 LO SO SHING LAMMA SOUTH 14 LUK CHAU LAMMA SOUTH 15 MO TAT LAMMA SOUTH 16 MO TAT WAN LAMMA SOUTH 17 PO TOI LAMMA SOUTH 18 SOK KWU WAN LAMMA SOUTH 19 TUNG O LAMMA SOUTH 20 YUNG SHUE HA LAMMA SOUTH 21 CHUNG HAU MUI WO 2 22 LUK TEI TONG MUI WO 23 MAN KOK TSUI MUI WO 24 MANG TONG MUI WO 25 MUI WO KAU TSUEN MUI WO 26 NGAU KWU LONG MUI WO 27 PAK MONG MUI WO 28 PAK NGAN HEUNG MUI WO 29 TAI HO MUI WO 30 TAI TEI TONG MUI WO 31 TUNG WAN TAU MUI WO 32 WONG FUNG TIN MUI WO 33 CHEUNG SHA LOWER VILLAGE SOUTH LANTAU 34 CHEUNG SHA UPPER VILLAGE SOUTH LANTAU 35 HAM TIN SOUTH LANTAU 36 LO UK SOUTH LANTAU 37 MONG TUNG WAN SOUTH LANTAU 38 PUI O KAU TSUEN (LO WAI) SOUTH LANTAU 39 PUI O SAN TSUEN (SAN WAI) SOUTH LANTAU 40 SHAN SHEK WAN SOUTH LANTAU 41 SHAP LONG SOUTH LANTAU 42 SHUI HAU SOUTH LANTAU 43 SIU A CHAU SOUTH LANTAU 44 TAI A CHAU SOUTH LANTAU 3 45 TAI LONG SOUTH LANTAU 46 TONG FUK SOUTH LANTAU 47 FAN LAU TAI O 48 KEUNG SHAN, LOWER TAI O 49 KEUNG SHAN, -

PWSC(2002-03)20 on 8 May 2002
For discussion PWSC(2002-03)20 on 8 May 2002 ITEM FOR PUBLIC WORKS SUBCOMMITTEE OF FINANCE COMMITTEE HEAD 705 – CIVIL ENGINEERING Civil Engineering – Land Development 660CL – Site formation, construction of associated infrastructure and provision of government, institution and community facilities for an international theme park on Lantau Island Members are invited to recommend to Finance Committee – (a) the upgrading of part of 660CL, entitled “Infrastructure for Penny’s Bay Development, Package 3 and Penny’s Bay Reclamation Stage 2”, to Category A at an estimated cost of $2,375.9 million in money-of-the-day prices; and (b) the retention of the remainder of 660CL in Category B. PROBLEM We need to provide the necessary infrastructure and government, institution and community (GIC) facilities to support the development of Hong Kong Disneyland (HKD) Phase 1 at Penny’s Bay on Lantau Island. We also need to provide land for the future development of HKD Phase 2. /PROPOSAL ..... PWSC(2002-03)20 Page 2 PROPOSAL 2. The Director of Civil Engineering (DCE), with the support of the Secretary for Economic Services, proposes to upgrade part of 660CL to Category A at an estimated cost of $2,375.9 million in money-of-the-day (MOD) prices for the construction of infrastructure and GIC facilities to serve HKD Phase 1 and the reclamation works for the future development of HKD Phase 2. PROJECT SCOPE AND NATURE 3. The scope of the part of 660CL which we now propose to upgrade to Category A comprises - (a) construction of a section of Road P2 about 1.8 kilometres -

1989 Tiananmen Square Massacre
EBSCOhost Page 1 of 6 Record: 1 Title: 1989 TIANANMEN SQUARE MASSACRE. Source: Junior Scholastic; 3/2/2009, Vol. 111 Issue 13, p6-10, 5p Document Type: Article Subject Terms: CHINA -- History -- Tiananmen Square Incident, 1989 Geographic Terms: BEIJING (China) CHINA Report Available Abstract: The article traces the 1989 Tiananmen Square massacre in Beijing, China. Lexile: 940 Full Text Word Count:1821 ISSN: 00226688 Accession Number: 37332978 Database: Middle Search Plus Section: World 1989 TIANANMEN SQUARE MASSACRE TWENTY YEARS AGO THIS SPRING, CHINA'S ARMY FORCED A BLOODY CONFRONTATION AGAINST PEACEFUL STUDENT DEMONSTRATORS On May 29, 1989, a 27-foot-tall foam-and-papier-mâché statue appeared in Tiananmen Square, the 100-acre heart of Beijing, China's capital. For more than a month, thousands of college students had occupied the square in defiance of China's Communist government. The towering figure, named the Goddess of Democracy, symbolized the students' goal of forcing the government to loosen its grip on the lives of China's 1.1 billion people. But to China's repressive rulers, the statue was an insult, perhaps more so because its features were not Chinese. Within days, they sent in army tanks to break up the protest. The result was one of the most violent crackdowns in China's history. Hundreds, perhaps thousands, of protesters were killed, and the statue was smashed--along with the hopes of many people across China. The protest had begun in April. Several thousand students, mourning the death of a progressive leader who had favored democratic reforms, marched through Beijing chanting pro-democracy slogans. -

Annual Report 2019 Corporate Information & Key Dates
QUICK FACTS 55 countries & territories 47,000+ employees worldwide 75M ft² land & facilities 10,000+ self-owned operating vehicles Cover Photo: A 33,000-kg air drum transported from Italy to a refinery in Russia by sea & road freight in 60 days. (computer-processed image) CONTENTS 02 Corporate Information & Key Dates 03 Financial Highlights 05 2015-2019 Financial Summary 06 Logistics Facilities GLOBAL NETWORK 13 Chairman’s Statement 14 Management Discussion and Analysis 14 Results Overview 15 Business Review 19 Financial Review 20 Staff and Remuneration Policies 21 Environmental, Social and Governance Report 34 Awards and Citations CHINA FOCUS 40 Corporate Governance Report 60 Directors and Senior Management 73 Report of Directors 99 Independent Auditor’s Report 108 Statement of Accounts 203 Definitions ASIA SPECIALIST 1 ANNUAL REPORT 2019 CORPORATE INFORMATION & KEY DATES BOARD OF DIRECTORS AUDITOR Executive Directors PricewaterhouseCoopers Mr KUOK Khoon Hua (Chairman) Certified Public Accountants and Registered PIE Auditor Mr MA Wing Kai William (Group Managing Director) Mr CHEUNG Ping Chuen Vicky LEGAL ADVISER Mr NG Kin Hang Davis Polk & Wardwell Non-executive Director REGISTERED OFFICE Ms TONG Shao Ming Victoria Place, 5th Floor, 31 Victoria Street Hamilton HM 10, Bermuda Independent Non-executive Directors Ms KHOO Shulamite N K CORPORATE HEADQUARTERS AND Ms WONG Yu Pok Marina PRINCIPAL PLACE OF BUSINESS IN HONG KONG Mr YEO Philip Liat Kok 16/F, Kerry Cargo Centre, 55 Wing Kei Road Mr ZHANG Yi Kevin Kwai Chung, New Territories, Hong -
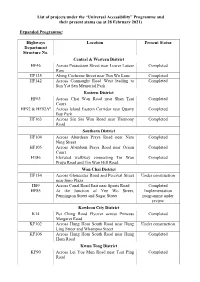
List of Projects Under the “Universal Accessibility” Programme and Their Present Status (As at 28 February 2021)
List of projects under the “Universal Accessibility” Programme and their present status (as at 28 February 2021) Expanded Programme: Highways Location Present Status Department Structure No. Central & Western District HF46 Across Possession Street near Lower Lascar Completed Row HF135 Along Cochrane Street near Tun Wo Lane Completed HF142 Across Connaught Road West leading to Completed Sun Yat Sen Memorial Park Eastern District HF63 Across Chai Wan Road near Shan Tsui Completed Court HF92 & HF92A# Across Island Eastern Corridor near Quarry Completed Bay Park HF163 Across Siu Sai Wan Road near Harmony Completed Road Southern District HF104 Across Aberdeen Praya Road near Nam Completed Ning Street HF105 Across Aberdeen Praya Road near Ocean Completed Court H186 Elevated walkway connecting Tin Wan Completed Praya Road and Tin Wan Hill Road Wan Chai District HF154 Across Gloucester Road and Percival Street Under construction near Sino Plaza HS9 Across Canal Road East near Sports Road Completed HF85 At the Junction of Yee Wo Street, Implementation Pennington Street and Sugar Street programme under review Kowloon City District K14 Pui Ching Road Flyover across Princess Completed Margaret Road KF102 Across Hung Hom South Road near Hung Under construction Ling Street and Whampoa Street KF106 Across Hung Hom South Road near Hung Completed Hom Road Kwun Tong District KF90 Across Lei Yue Mun Road near Tsui Ping Completed Road Highways Location Present Status Department Structure No. KF109 Across Shun Lee Tsuen Road near Shun Completed Lee Estate -

Inventory of the Collection Chinese People's Movement, Spring 1989 Volume Ii: Audiovisual Materials, Objects and Newspapers
International Institute of Social History www.iisg.nl/collections/tiananmen/ INVENTORY OF THE COLLECTION CHINESE PEOPLE'S MOVEMENT, SPRING 1989 VOLUME II: AUDIOVISUAL MATERIALS, OBJECTS AND NEWSPAPERS at the International Institute of Social History (IISH) International Institute of Social History www.iisg.nl/collections/tiananmen/ For a list of the Working Papers published by Stichting beheer IISG, see page 181. International Institute of Social History www.iisg.nl/collections/tiananmen/ Frank N. Pieke and Fons Lamboo INVENTORY OF THE COLLECTION CHINESE PEOPLE'S MOVEMENT, SPRING 1989 VOLUME II: AUDIOVISUAL MATERIALS, OBJECTS AND NEWSPAPERS at the International Institute of Social History (IISH) Stichting Beheer IISG Amsterdam 1991 International Institute of Social History www.iisg.nl/collections/tiananmen/ CIP-GEGEVENS KONINLIJKE BIBLIOTHEEK, DEN HAAG Pieke, Frank N. Inventory of the Collection Chinese People's Movement, spring 1989 / Frank N. Pieke and Fons Lamboo. - Amsterdam: Stichting beheer IISG Vol. II: Audiovisual Materials, Objects and Newspapers at the International Institute of Social History (IISH). - (IISG-werkuitgaven = IISG-working papers, ISSN 0921-4585 ; 16) Met reg. ISBN 90-6861-060-0 Trefw.: Chinese volksbeweging (collectie) ; IISG ; catalogi. c 1991 Stichting beheer IISG All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher. Niets uit deze uitgave mag worden vermenigvuldigd en/of openbaar worden gemaakt door middel van druk, fotocopie, microfilm of op welke andere wijze ook zonder voorafgaande schriftelijke toestemming van de uitgever. Printed in the Netherlands International Institute of Social History www.iisg.nl/collections/tiananmen/ TABLE OF CONTENTS Table of Contents v Preface vi 1. -

Mon Tue Wed Thu Fri Sat Sun 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
WWF - DDC Location Plan May-2018 Mon Tue Wed Thu Fri Sat Sun 1 2 3 4 5 6 Team A Day-Off Tsuen Wan Plaza Diamond Hill MTR Exit A2 Lau Sin Street, Tin Hau King Man Street, Sai Kung (Near Sai Kung Library) Day-Off Hang Seng Bank Head Office, Central New Jade Shopping Centre, Chai Wan New Jade Shopping Centre, Chai Wan Team B Shatin Plaza Shatin Plaza Shatin Plaza (Near Footbridge) (Near Footbridge) (Near Footbridge) Hang Seng Bank Head Office, Central New Jade Shopping Centre, Chai Wan Team C Canal Road East Bridge, Causeway Bay Hong Kong MTR Station Yuen Long MTR Station Yuen Long MTR Station (Near Footbridge) (Near Footbridge) Hang Seng Bank Head Office, Central Team D Wu Kai Sha MTR Station Exit B Canal Road East Bridge, Causeway Bay Yuen Long MTR Station Tsuen Wan Plaza (Near Footbridge) Tsuen Wan Plaza (Near Footbridge) (Near Footbridge) World Wide House, Central World Wide House, Central Team E Tuen Mun MTR Station Wu Kai Sha MTR Station Exit B Canal Road East Bridge, Causeway Bay Tsuen Wan Plaza (Near Footbridge) (Near Footbridge) (Near Footbridge) World Wide House, Central Team F Causeway Bay Plaza 1 (Near Footbridge) Tuen Mun MTR Station Wu Kai Sha MTR Station Exit B Chai Wan MTR Station Chai Wan MTR Station (Near Footbridge) Team G Admiralty MTR Station Exit A Causeway Bay Plaza 1 (Near Footbridge) Tuen Mun MTR Station Chai Wan MTR Station Austin MTR Station Exit B (Near Footbridge) Austin MTR Station Exit B (Near Footbridge) Team H Tai Wai MTR Station Exit D Admiralty MTR Station Exit A Causeway Bay Plaza 1 (Near Footbridge) Austin