A Baseline Study to Evaluate the Estrogenic Activities Using In-Vitro Yeast Estrogenic Screen in the Selected Ramsar Sites and Creek Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
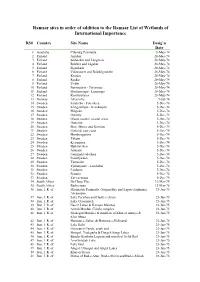
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Table of Contents
Environmental and Social Management Framework (ESMF) Draft Pakistan Hydro-Meteorological and DRM Services Project Pakistan Meteorological Department National Disaster Management Authority Pakistan Hydro-Meteorological and DRM Services Project Executive Summary Background Climate change is expected to have an adverse impact on Pakistan, as it ranks 7th on the climate risk index. It continues to be one of the most flood-prone countries in the South Asia Region (SAR); suffering US$18 billion in losses between 2005 and 2014 (US$10.5 billion from the 2010 floods alone), equivalent to around 6% of the federal budget. Hydromet hazards have been coupled with rapid population growth and uncontrolled urbanization, leading to a disproportionate and growing impact on the poor. To build on recent development gains, increase economic productivity, and improve climate resilience, it will be critical to improve the quality and accessibility of weather, water, and climate information services. Climate-resilient development requires stronger institutions and a higher level of observation, forecasting, and service delivery capacity; these could make a significant contribution to safety, security, and economic well-being. The Pakistan Hydro- Meteorological and DRM Services Project (PHDSP) expects to improve hydro- meteorological information and services, strengthen forecasting and early warning systems, and improve dissemination of meteorological and hydrological forecasts, warnings and advisory information to stakeholders and end-users and strengthen the existing disaster risk management (DRM) capacity and services of the National Disaster Management Authority (NDMA). Project Description The project has three main components and will be implemented over a period of five years. Component 1: Hydro-Meteorological and Climate Services The objective of this component is to improve the capability and thereby performance of the PMD to understand and make use of meteorological and hydrological information for decision making. -

WETLANDS in PAKISTAN: WHAT IS HAPPENING to THEM? By: Abdul Aleem Chaudhry Ph.D
World Environment Day – June 2010 49 WETLANDS IN PAKISTAN: WHAT IS HAPPENING TO THEM? By: Abdul Aleem Chaudhry Ph.D. Director General Wildlife and Parks Punjab (Retired) Abstract Pakistan, despite having an arid climate, supports over 780,000 ha of wetlands covering 9.7% of the total land area, with 225 nationally significant wetlands, of which 19 have been recognised as Ramsar sites of global significance. Wetland types represent the passage of the Indus River from the glaciers and high alpine lakes, through riverine and freshwater lakes to the coastal wetlands of the Indus Delta. These wetlands provide often unrecognised benefits and services, such as provisioning - food and fibre production - regulating services such as water balance, groundwater recharge, flood mitigation and storm protection; cultural and social functions such as sacred and religious importance; providing recreation and tourism opportunities; and supporting functions such as soil formation and sediment retention. Main threats to wetlands include shortages of water to maintain the wetlands, poor water quality from increasing pollution, change in land use, encroachment and over- exploitation of natural resources, such as fish and wildlife. Most often the over- exploitation is driven by the lack of alternative livelihoods so that poor communities may have no option. The underlying causes of these direct threats are related to the perception that wetland natural resources are part of an open- access system. Management of the natural resources, if it exists at all, is usually ineffective and penalties for illegal or inappropriate resource-use are often not significant enough to be prohibitive. These inappropriate practices generally stem from policy shortcomings, legal gaps and inconsistencies, failure to enforce regulations, and institutional overlap of responsibilities for management of wetlands and lack of coordination. -

Annotated List of Wetlands of International Importance Pakistan
Ramsar Sites Information Service Annotated List of Wetlands of International Importance Pakistan 19 Ramsar Site(s) covering 1,343,807 ha Astola Island Site number: 1,063 | Country: Pakistan | Administrative region: Balochistan Province Area: 5,000 ha | Coordinates: 25°07'N 63°52'E | Designation dates: 10-05-2001 View Site details in RSIS Astola (Haft Talar) Island. 10/05/01. Balochistan. 5,000 ha. 25°07'N 063°52'E. An uninhabitated island about six km in length, some 25 km south of the desert coast of Balochistan. It is the only significant offshore island along the north coast of the Arabian Sea, and as such maintains the genetic and ecological diversity of the area. The endangered Green turtle (Chelonia mydas) and possibly the Hawksbill turtle (Eretmochelys imbracata) nest on the beach at the foot of cliffs, and it is a very important area for endemic reptiles such as the viper Echis carinatus astoli. The island is said to have an aura of mystery and is venerated by Hindus; there are architectural remains of an ancient temple to the Hindu goddess Kali Devi, as well as a prayer yard constructed for a Muslim saint associated with oceans. It serves as a base for fishermen between September and May, but is unfrequented during the period of rough seas and high tides. Feral cats originally introduced by fishermen to control the endemic rodent population pose an increasing threat to birds' nesting and breeding sites. Reprint of the RIS. Ramsar site no. 1063. Most recent RIS information: 2001. Chashma Barrage Site number: 816 | Country: Pakistan | Administrative region: Punjab Province Area: 34,099 ha | Coordinates: 32°25'N 71°22'E | Designation dates: 22-03-1996 View Site details in RSIS Chashma Barrage. -

The Case of Nurerri and Jubho Lagoons, Pakistan
Linking Property Rights with Environmental Changes: The case of Nurerri and Jubho Lagoons, Pakistan by Sajida Sultana A thesis presented to the University of Waterloo in fulfilment of the thesis requirement for the degree of Master of Environmental Studies in Sustainability Management-Water Waterloo, Ontario, Canada, 2015 Sajida Sultana 2015 i Author's Declaration “I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public.” ii Abstract Coastal lagoons play a vital role in supporting human well-being and the conservation of unique biological resources. They are crucial for the protection of the coastline from extreme events like floods, and for providing diverse livelihood opportunities to people. However, coastal lagoons face a range of threats from multiple drivers at local, regional and global scales, and those drivers are both anthropogenic and climatic in nature. In this thesis, I use a commons approach to examine changes in property rights regime in the Nurerri and Jubho lagoons of Pakistan to better understand their linkages with processes of environmental change. Both Nurerri and Jubho Lagoons are designated Ramsar sites inthe Indus delta of Pakistan, which is the world’s fifth largest delta system. The entire region, which includes a number of other important wetlands, has undergone serious degradation over the past three decades. This research considers the history of changes in the property rights regime in relation to the processes of environmental change. -

Ramsar Sites in Pakistan
Ramsar Advisory Mission Pakistan 29 October – 5 November, 2012 What are wetlands? Wetlands are areas of marsh, fen, peatland or water, whether natural or artificial, permanent or temporary, with water that is static or flowing, fresh, brackish or salt, including areas of marine water the depth of which at low tide does not exceed six metres. Inland Wetlands Glaciers, lakes, marshes, rivers, streams, aquifers. Coastal Wetlands © Ms. Ikram Qasim Mangroves, tidal flats, sea grass beds, coral reefs. Human-made wetlands: Rice fields, fish ponds, reservoirs, ditches and canals. Wetland ecosystem services Natural infrastructures: Provisioning: water, food, • important part of the water cycle biodiversity, and hydroelectricity • stores and delivers water Supporting: soil formation, nutrient cycling, Regulating: floods and drought Culture: recreation, spiritual, education, tourism The Ramsar Convention on Wetlands • Adopted on 2 February, 1971 • First of the modern intergovernmental environmental agreements Mission The conservation and wise use of wetlands through national actions, and international cooperation as a contribution towards achieving sustainable development. • 163 Contracting Parties. • Pakistan joined in 1976 The 3 implementation “pillars” of the Convention 1. “Wise use” of wetland This is at the heart of the Convention, the sustainable use of wetlands and their resources for the benefit of humankind. 2. Designation of Wetlands of international © Ms. Ikram Qasim Importance Designation of priority wetlands as Wetlands of International Importance (Ramsar Sites), and to ensure their conservation and wise use. 3. International cooperation International cooperation for shared wetlands and © Ms. M. Kodami © Dr. M. Fouda their resources, e.g. water, migratory species. © Ms. Ikram Qasim Ramsar Sites in Pakistan 1. -

Fractional Land Cover Classification and Temporal Change Evaluation
Fractional Land Cover Classification and Temporal Change Evaluation of Deh Akro-II Wetland Complex and Chotiari Wetland Complex, Sindh, Pakistan, Using Google Earth Engine Samawia Rizwan1*; Dr. Khalid Mahmood2; Dr. Sajid Rashid Ahmad3; Dr. Shafiq Ur Rehman4 1*Lecturer, Centre of Geographic Information System, University of the Punjab, Lahore, Pakistan. 1*[email protected] 2Assistant Professor, Department of Space Science, University of the Punjab, Lahore, Pakistan. [email protected] 3Principal, Professor CEES, Collage of Earth and Environmental Sciences, University of the Punjab, Lahore, Pakistan. [email protected] 4Department of Botany, University of Okara, Pakistan. [email protected] Abstract Wetlands are one of the most important and rich eco system. Deh akro II wetland complex is unique inland type of wetlands comprise of 35 wetlands in middle of Nara desert on bank of Nara Canal. They face a lot of degradation because of anthropogenic activities in the surrounding areas and lack of rainfall in last 2 decades. Chotiari wetland complex located in south east of Deh akro II wetland complex, it comprises of several fresh water lakes converted into reservoir in year 2003 for better irrigation purposes. This conversion of wetlands into reservoir does not did very well for surrounding agricultural lands and natural vegetation. So in this study two technique of Fractional cover mapping were used to classify three types of land covers in both study areas. Temporal analysis was performed using the Landsat 7 ETM+ image of year 2000 and Landsat 8 OLI image of year 2018. For better results NDVI, EVI and NDWI were also calculated. For Deh akro II wetland complex Kappa accuracy statistics for year 2000 is 84% and for year 2018 its 87%. -
Ramsar "Wetlands of International Importance" with Lake Habitats
Ramsar "Wetlands of International Importance" with lake habitats The Ramsar Convention is an intergovernmental treaty adopted in 1971 which relates to "all areas where water is the primary factor controlling the environment and associated plant and animal life", thus including lakes. Wetlands are listed as "wetlands of international importance" after being nominated by their resident country and meeting at least one of eight criteria set out under the Ramsar Convention. Over 300 Ramsar sites are primarily lake habitats, and another 350 include at least some lake habitat. The chart below is based on Ramsar data obtained from Wetlands International, Oct. 2002. Ramsar sites in bold indicate that the site is primarily lake habitat; Ramsar sites not appearing in bold are sites at which some lake habitat occurs, but it is not the main habitat of the site. Country Ramsar site Lake habitat type Algeria Chott El Hodna Intermittent saline Algeria Lac des Oiseaux, ou Garaet et Touyour Permanent fresh Algeria Lac Oubeïra Permanent fresh Algeria Lac Tonga Permanent fresh Algeria Sebkha d'Oran Intermittent saline Algeria La Vallée d'Iherir Intermittent fresh Algeria Chott Merrouane et Oued Khrouf Permanent saline Algeria Marais de la Macta Permanent saline Algeria Oasis de Tamantit et Sid Ahmed Timmi Intermittent saline Algeria Oasis de Ouled Saïd Intermittent saline Argentina Laguna Blanca Permanent fresh Argentina Lagunas y Esteros del Iberá Permanent fresh Argentina Lagunas de Guanacache Intermittent fresh Argentina Bañados del Río Dulce y Laguna -
Recent Observations on the Distribution and Status of Wildlife of Balochis Tan
Canadian Journal of Pure and Applied Sc iences Vol. 13, No. 2, pp. 4813 - 4846 , June 2019 Online ISSN: 1920 - 3853; Print ISSN: 1715 - 9997 Available online at www.cjpas.net Special Article RECENT OBSERVATIONS ON THE DISTRIBUTION AND STATUS OF WILDLIFE OF BALOCHIS TAN * Syed Ali Ghalib 1 , Muhammad Zaheer Khan 1 , Ubaid Ullah 1 , Roohi Kanwal 1 , Afsheen Zehra 1 , Saima Siddiqui 1 , Dar a khshan Abbas 1 , Ghazala Yasmeen 1 , Babar Hussai n 2 and Abdur Raz z aq Khan 3 1 Department of Zoology, University of Karachi, Karachi - 75270 2 IUCN - Pakistan, 1 Bath Island Road Karachi 3 Halcrow Pakistan (Pvt) Limited, Karachi ABSTRACT Field surveys were undertaken from 2015 to 2019 in ma jor ecological zones of the Balo ch istan province to collect data o n the distribution and status of its wildlife. Based on five years field observations, 92 species of ma mmals, 373 species of birds, 120 species of reptiles and seven species of amphibians were documented . The p resent study gives an update list of Wildlife Protected Areas of the Province, the list of Threatened/Near - threatened species, the important wildlife hotspots, important ecotourism sites and key/peculiar of wildlife species. In regard to the Threatened/Nae r - threatened species of wildlife, among mammals four species are Endangered (EN), eight species are Vulnerable (VU) and three species are Near - threatened (NT) . Among birds, two species are Critically Endangered (CR), four species are EN, three species are Vulnerable and 15 species are Near - threatened. Among reptiles, one species is Critically Endangered, on e species is Endangered and six species are Vulnerable. -

Ramsar COP7 DOC
Ramsar COP7 DOC. 20.1 “People and Wetlands: The Vital Link” 7th Meeting of the Conference of the Contracting Parties to the Convention on Wetlands (Ramsar, Iran, 1971), San José, Costa Rica, 10-18 May 1999 Technical Session V: The framework for regional and international cooperation regarding wetlands Paper 1 Shared Wetlands and River Basins of the World: Preliminary Findings of a GIS Analysis Report prepared in support of Ramsar COP7 Technical Session V The frameworks for regional and international cooperation by Dr Brian Groombridge, World Conservation Monitoring Centre Contents page Executive summary 1 Ramsar sites and international borders 3 Ramsar sites and international catchment basins 5 Ramsar sites and basin vulnerability 7 Wetlands areas and international catchment basins 8 Recommendations for further study 10 References 11 Annex 1: Catchment basin boundaries 12 Annex 2 : Key to country codes 12 Tables 1-7 15 EXECUTIVE SUMMARY 1. The purpose of this project was to highlight opportunities for international cooperation over management of wetland sites. The study was based on two assumptions: i. that cross-border, near-border or coastal sites may be at risk from transboundary effects, and ii. that sites within shared catchment basins may be similarly at risk, particularly where most of the basin and territory of more than one country are located upstream of the site. Geographic Information System (GIS) technology was used to analyse sets of spatial data with respect to these assumptions. Ramsar COP7 DOC. 20.1, Shared wetlands, page 2 2. Of a total 955 Ramsar sites included in this study, 92 (10%) are located in part or entirely within 10km of an international border, and thus in principle may be subject to transboundary influences, such as water abstraction or drainage. -

Report on the Ramsar Advisory Mission (# 431) to the Indus Dolphin River Ramsar Site and Associated Floodplains
REPORT ON THE RAMSAR ADVISORY MISSION (# 431) TO THE INDUS DOLPHIN RIVER RAMSAR SITE AND ASSOCIATED FLOODPLAINS 29 October – 5 November, 2012 Report of RAM to the Indus River, Pakistan (RAM 431) | 1 CONTENTS 1. Background 6 a. The Indus River 6 b. Management of the Indus River and its resources 7 c. Flood disaster management in Pakistan 7 d. Rainfall patterns in Pakistan 7 e. Floods along the Indus River 8 f. 2010 Floods 8 g. Background to the Ramsar Advisory Mission 8 2. Floodplains in risk management 10 a. Floodplains and their value 10 b. Management of floodplains 11 3. Lessons learnt from floodplain management along the Yangtze River, P.R. China 12 a. The Yangtze River 12 b. Floods in the Yangtze River 12 c. Floods in the 1990’s 13 d. Lessons learnt from the 1998 Yangtze floods 13 e. Integrated River Basin Management in the Yangtze River 16 f. Local legislation on wetland conservation 17 g. Developing alternative solutions for local development 17 h. Summary 18 4. Results of meetings 21 a. Flood disaster management in Pakistan 21 b. Future management of floods 21 c. Conservation management of Ramsar Sites along the Indus River floodplains 22 d. Conservation management of flooded forests along the Indus River floodplains 23 5. Discussion 24 a. RAM Objective 1: To devise a workable and cost effective strategy for wise-use of the 24 floods b. RAM Objective 2: To make recommendations for alteration in the prevailing flood 27 control strategy so as to obtain maximum benefit from the flood water c. -

CBD Strategy and Action Plan 8
Pakistan National Biodiversity Strategy and Action Plan for achieving Aichi Biodiversity Targets and Sustainable Development Goals Government of Pakistan 2017 - 2030 – NBSAP TABLE OF CONTENTS TABLE OF CONTENTS ................................................................................................................. i Executive Summary ......................................................................................................................... i I. INTRODUCTION .............................................................................................................. 12 1. Context ............................................................................................................................... 12 2. Purpose of National Biodiversity Strategy and Action Plan .............................................. 13 3. Valuation of Biodiversity & Ecosystem Services and its Contribution to Human Well- being ................................................................................................................................... 14 4. Causes and Trends of Biodiversity Loss ............................................................................ 14 4.1. Population Growth ......................................................................................................... 15 4.2. Policy, Planning, and Institutional Shortfalls ................................................................ 15 4.3. Land Use -Degradation and Desertification .................................................................