British Aneurysm Nimodipine Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Migraine Prophylaxis: Which Drugs Work and Which Ones Don't
Migraine Prophylaxis: Which Drugs Work and Which Ones Don’t Hans-Christoph Diener, MD Department of Neurology, University Hospital Essen, Essen, Germany. J Gen Intern Med 28(9):1125–6 investigated in two properly powered and conducted studies DOI: 10.1007/s11606-013-2469-2 and found not to be effective compared to placebo.3, 4 Adding © Society of General Internal Medicine 2013 data from poorly controlled and underpowered studies in a meta-analysis, as in this paper, gives the wrong impression that he paper by Shamliyan et al.1 in this issue of JGIM is a very nimodipine is as effective in migraine prevention as propran- T important contribution to headache research. The authors olol. Another example illustrated in this meta-analysis involves conducted a systematic literature review of drug treatment for the gabapentin. Most of the published trials are poorly conducted, underpowered, or have manipulated statistical analyses, such as prevention of episodic migraine. They analysed randomised 5 controlled trials (RCTs) and performed meta-analyses where modified intention-to-treat analyses. The meta-analysis in this appropriate. The two important outcomes examined in the paper indicated possible efficacy. Yet, in the time between the analysis are a ≥ 50 % reduction in migraine frequency and submission and publication of this paper, a recent well-powered adverse events leading to treatment discontinuation. dose finding trial was published investigating four doses of gabapentin as compared with placebo for migraine prevention. 6 What are the strengths of this paper? This trial showed no benefit for gabapentin. Another problem inherent in meta-analyses that this paper The authors are experts in this kind of analysis. -

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -

Migraine Headache Prophylaxis Hien Ha, Pharmd, and Annika Gonzalez, MD, Christus Santa Rosa Family Medicine Residency Program, San Antonio, Texas
Migraine Headache Prophylaxis Hien Ha, PharmD, and Annika Gonzalez, MD, Christus Santa Rosa Family Medicine Residency Program, San Antonio, Texas Migraines impose significant health and financial burdens. Approximately 38% of patients with episodic migraines would benefit from preventive therapy, but less than 13% take prophylactic medications. Preventive medication therapy reduces migraine frequency, severity, and headache-related distress. Preventive therapy may also improve quality of life and prevent the progression to chronic migraines. Some indications for preventive therapy include four or more headaches a month, eight or more headache days a month, debilitating headaches, and medication- overuse headaches. Identifying and managing environmental, dietary, and behavioral triggers are useful strategies for preventing migraines. First-line med- ications established as effective based on clinical evidence include divalproex, topiramate, metoprolol, propranolol, and timolol. Medications such as ami- triptyline, venlafaxine, atenolol, and nadolol are probably effective but should be second-line therapy. There is limited evidence for nebivolol, bisoprolol, pindolol, carbamazepine, gabapentin, fluoxetine, nicardipine, verapamil, nimodipine, nifedipine, lisinopril, and candesartan. Acebutolol, oxcarbazepine, lamotrigine, and telmisartan are ineffective. Newer agents target calcitonin gene-related peptide pain transmission in the migraine pain pathway and have recently received approval from the U.S. Food and Drug Administration; how- ever, more studies of long-term effectiveness and adverse effects are needed. The complementary treatments petasites, feverfew, magnesium, and riboflavin are probably effective. Nonpharmacologic therapies such as relaxation training, thermal biofeedback combined with relaxation training, electromyographic feedback, and cognitive behavior therapy also have good evidence to support their use in migraine prevention. (Am Fam Physician. 2019; 99(1):17-24. -

Original Article Comparison of Therapeutic Effects of Two Ccbs on Glaucoma and Analysis of Their Possible Mechanisms
Int J Clin Exp Med 2017;10(7):10560-10564 www.ijcem.com /ISSN:1940-5901/IJCEM0056272 Original Article Comparison of therapeutic effects of two CCBs on glaucoma and analysis of their possible mechanisms Tao Liang, Lingyun Zhang, Yanhua Gao, Yanru Xiang, Yan Gao Department of Ophthalmology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China Received April 26, 2017; Accepted May 26, 2017; Epub July 15, 2017; Published July 30, 2017 Abstract: Objective: To respectively compare the therapeutic effects of nimodipine and nifedipine on glaucoma, and then analyze the possible protective effects of these two calcium channel blockers (CCBs) on glaucomatous retinal ganglion cells (RGCs). Methods: Fifty-four patients with glaucoma were divided into control group (n=15), treat- ment group 1 (n=20) and treatment group 2 (n=19) in accordance with a random number table. General clinical treatment of glaucoma was performed in all three groups, while nimodipine was applied in treatment group 1 and nifedipine was applied in treatment group 2. The therapeutic effects and incidence of adverse reactions (intraocular pressure (IOP), eyesight, retinal light sensitivity, progressive visual field damage and adverse drug reaction) were compared among the three groups. Results: There were no significant differences in IOP and eyesight before and after treatment among the three groups (P>0.05). The retinal light sensitivity in control group began to decline from the sixth month after treatment, which was significantly different from treatment group 1 and treatment group 2 (P=0.03; P=0.04). The survival curve of visual field damage indicated that the visual field damage in control group was obviously more serious than that in the two treatment groups with the increase of sick time (P=0.03). -

Cyproheptadine Versus Propranolol in the Prevention of Migraine
Original Article Cyproheptadine versus propranolol in the prevention of migraine headaches in children Bahador Asadi1, Fariborz Khorvash2, Abolfazl Najaran3, Farzin Khorvash4 ABSTRACT Objective: There are conflicting results on the efficacy of propranolol and cyproheptadine in the prevention of migraine headaches in children. Therefore, in this study, we evaluated the efficacy of propranolol versus cyproheptadine in the prevention of migraine headaches. Methodology: This was a randomized, double-blind trial. Sixty children aged 8-15 yrs with migraine headaches were randomized to be treated with either propranolol (40-80mg per day) or cyproheptadine (8-12mg per day) for 4 weeks. The patients were requested to record the severity and duration of their headaches during a 2-week period before starting the intervention. The patients were followed at 2-week intervals for a period of 1 month after starting treatment. The headache diary was analyzed for each patient and was compared with baseline using SPSS software and statistical tests including the student’s t-test. Results: Out of 60 patients at baseline, nine patients in the cyproheptadine group and six patients in the propranolol group did not appear at the appropriate time for follow-up visits and therefore were excluded from the study. The mean age in the cyproheptadine group was 11.9 ± 2.23 years and in the propranolol group was 10.7 ± 2.33 years. Based on the diaries, the results showed that propranolol and cyproheptadine decreased headaches by 54.61% and 70.53% (p < 0.05), respectively, at the end of four weeks of treatment. Conclusion: Overall, the results of our study suggest that cyproheptadine is a good choice for prevention of migraine headache in pediatric group although more prolonged study with higher number of the patient is recommended. -

Ep 0932416 B1
Europäisches Patentamt *EP000932416B1* (19) European Patent Office Office européen des brevets (11) EP 0 932 416 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: A61K 45/06, A61K 31/485, of the grant of the patent: A61K 31/165, A61K 31/135, 22.06.2005 Bulletin 2005/25 A61K 31/00, A61K 31/275 (21) Application number: 97910772.9 (86) International application number: PCT/US1997/017828 (22) Date of filing: 06.10.1997 (87) International publication number: WO 1998/015275 (16.04.1998 Gazette 1998/15) (54) METHOD AND POTENTIATED COMPOSITION FOR TREATING MIGRAINE VERFAHREN UND POTENZIERTE ZUSAMMENSETZUNG ZUR BEHANDLUNG VON MIGRÄNE THERAPIE ET COMPOSITION PHARMACEUTIQUE POTENTIALISEE EFFICACES CONTRE LA MIGRAINE (84) Designated Contracting States: • DHAVARE ET AL: "Effect of Drugs Influencing AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC Central 5-HT Mechanisms on NL PT SE Amantadine-Induced Stereotyped Behaviour in the Rat" INDIAN JOURNAL PHYSIOL. (30) Priority: 09.10.1996 US 727923 PHARMACOL., vol. 27, no. 1, 1983, pages 19-24, 24.10.1996 US 736370 XP002058885 • MAJ T ET AL: "Antagonistic Effect of (43) Date of publication of application: Cyproheptadine on Neuroleptic-Induced 04.08.1999 Bulletin 1999/31 Catalepsy" PHARMACOLOGY BIOCHEMISTRY AND BEHAVIOR, vol. 3, no. 1, 1975, pages 25-27, (73) Proprietor: Algos Pharmaceutical Corporation XP002058886 Neptune, NJ 07753 (US) • SKUZA ET AL: "Memantine, Amantadine and L-Deprenyl Potentiate the Action of L-DOPA in (72) Inventor: CARUSO, Frank, S. Monoamine-Depleted Rats" JOURNAL OF Colts Neck, NJ 07722 (US) NEURAL TRANSMISSION, vol. -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -

Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
International Journal of Molecular Sciences Review Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives Diji Kuriakose and Zhicheng Xiao * Development and Stem Cells Program, Monash Biomedicine Discovery Institute and Department of Anatomy and Developmental Biology, Monash University, Melbourne, VIC 3800, Australia; [email protected] * Correspondence: [email protected] Received: 29 September 2020; Accepted: 13 October 2020; Published: 15 October 2020 Abstract: Stroke is the second leading cause of death and a major contributor to disability worldwide. The prevalence of stroke is highest in developing countries, with ischemic stroke being the most common type. Considerable progress has been made in our understanding of the pathophysiology of stroke and the underlying mechanisms leading to ischemic insult. Stroke therapy primarily focuses on restoring blood flow to the brain and treating stroke-induced neurological damage. Lack of success in recent clinical trials has led to significant refinement of animal models, focus-driven study design and use of new technologies in stroke research. Simultaneously, despite progress in stroke management, post-stroke care exerts a substantial impact on families, the healthcare system and the economy. Improvements in pre-clinical and clinical care are likely to underpin successful stroke treatment, recovery, rehabilitation and prevention. In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research. Keywords: stroke; pathophysiology; treatment; neurological deficit; recovery; rehabilitation 1. Introduction Stroke is a neurological disorder characterized by blockage of blood vessels. Clots form in the brain and interrupt blood flow, clogging arteries and causing blood vessels to break, leading to bleeding. -
PDF-Document
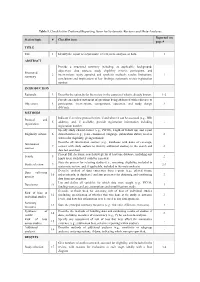
Table 1. Checklist for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Reported on Section/topic # Checklist item page # TITLE Title 1 Identify the report as a systematic review, meta-analysis, or both. 1 ABSTRACT Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and Structured 2 interventions; study appraisal and synthesis methods; results; limitations; 1 summary conclusions and implications of key findings; systematic review registration number. INTRODUCTION Rationale 3 Describe the rationale for the review in the context of what is already known. 1-2 Provide an explicit statement of questions being addressed with reference to Objectives 4 participants, interventions, comparisons, outcomes, and study design 2 (PICOS). METHODS Indicate if a review protocol exists, if and where it can be accessed (e.g., Web Protocol and 5 address), and, if available, provide registration information including - registration registration number. Specify study characteristics (e.g., PICOS, length of follow-up) and report Eligibility criteria 6 characteristics (e.g., years considered, language, publication status) used as 2 criteria for eligibility, giving rationale. Describe all information sources (e.g., databases with dates of coverage, Information 7 contact with study authors to identify additional studies) in the search and 2 sources date last searched. Present full electronic search strategy for at least one database, including any Search 8 2 limits used, such that it could be repeated. State the process for selecting studies (i.e., screening, eligibility, included in Study selection 9 2-3 systematic review, and, if applicable, included in the meta-analysis). Describe method of data extraction from reports (e.g., piloted forms, Data collection 10 independently, in duplicate) and any processes for obtaining and confirming 3 process data from investigators. -

Isolated Middle Cerebral Artery Dissection with Atherosclerosis: a Case Report
Neurology Asia 2018; 23(3) : 259 – 262 CASE REPORTS Isolated middle cerebral artery dissection with atherosclerosis: A case report Sang Hun Lee MD, Sun Ju Lee MD, Il Eok Jung MD, Jin-Man Jung MD Department of Neurology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Republic of Korea Abstract Isolated middle cerebral artery (MCA) dissection with atherosclerosis is a rare entity, and its clinical progression is not well known. We recently came across a case of isolated MCA dissection with atherosclerosis. A 62-year-old man presented to the emergency department with right-sided weakness and mild aphasia. Diffusion-weighted imaging (DWI) showed a multifocal infarction in the left MCA region, and perfusion Magnetic resonance imaging (MRI) detected a moderate time delay in the left MCA region. High-resolution MRI and transfemoral cerebral angiography revealed that the atherosclerotic plaque was accompanied by the dissecting intimal flap. Despite 40 days of antiplatelet therapy, the ischemic stroke recurred and the dissection did not heal. After stenting, the MCA and intracranial circulation revealed a widened lumen and improved flow across the dissection, and no embolic sequelae in the distal intracranial circulation. This case suggest that in MCA dissection with atherosclerosis, early stage intracranial stenting may be a better therapeutic strategy than medical treatment, to prevent recurrent cerebral infarction. Keywords: Middle cerebral artery dissection with atherosclerosis, middle cerebral artery dissection, atherosclerosis INTRODUCTION stroke and taking aspirin for 9 years, presented to the emergency department with right-sided Isolated middle cerebral artery (MCA) dissection weakness and mild aphasia. He had a history as a cause of stroke has been rarely reported.