Comparative Effectıveness of Novokinin, Perindopril And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Tolerability of the Direct Renin Inhibitor Aliskiren and Lisinopril in Patients with Severe Hypertension
Journal of Human Hypertension (2007) 21, 780–787 & 2007 Nature Publishing Group All rights reserved 0950-9240/07 $30.00 www.nature.com/jhh ORIGINAL ARTICLE A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension RH Strasser1, JG Puig2, C Farsang3, M Croket4,JLi5 and H van Ingen4 1Technical University Dresden, Heart Center, University Hospital, Dresden, Germany; 2Department of Internal Medicine, La Paz Hospital, Madrid, Spain; 31st Department of Internal Medicine, Semmelweis University, Budapest, Hungary; 4Novartis Pharma AG, Basel, Switzerland and 5Novartis Institutes for Biomedical Research, Cambridge, MA, USA Patients with severe hypertension (4180/110 mm Hg) LIS 3.4%). The most frequently reported AEs in both require large blood pressure (BP) reductions to reach groups were headache, nasopharyngitis and dizziness. recommended treatment goals (o140/90 mm Hg) and At end point, ALI showed similar mean reductions from usually require combination therapy to do so. This baseline to LIS in msDBP (ALI À18.5 mm Hg vs LIS 8-week, multicenter, randomized, double-blind, parallel- À20.1 mm Hg; mean treatment difference 1.7 mm Hg group study compared the tolerability and antihyperten- (95% confidence interval (CI) À1.0, 4.4)) and mean sitting sive efficacy of the novel direct renin inhibitor aliskiren systolic blood pressure (ALI À20.0 mm Hg vs LIS with the angiotensin converting enzyme inhibitor À22.3 mm Hg; mean treatment difference 2.8 mm Hg lisinopril in patients with severe hypertension (mean (95% CI À1.7, 7.4)). Responder rates (msDBPo90 mm Hg sitting diastolic blood pressure (msDBP)X105 mm Hg and/or reduction from baselineX10 mm Hg) were 81.5% and o120 mm Hg). -

Perindopril | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Perindopril This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Aceon [DSC] Brand Names: Canada AG-Perindopril; APO-Perindopril; Auro-Perindopril; BIO-Perindopril; Coversyl; JAMP-Perindopril; M-Perindopril Erbumine; MAR-Perindopril; MINT- Perindopril; NRA-Perindopril; PMS-Perindopril; Priva-Perindopril Erbumine; RIVA-Perindopril; SANDOZ Perindopril Erbumine; TEVA-Perindopril Warning Do not take if you are pregnant. Use during pregnancy may cause birth defects or loss of the unborn baby. If you get pregnant or plan on getting pregnant while taking this drug, call your doctor right away. What is this drug used for? It is used to treat high blood pressure. It is used to lower the risk of heart attack and death from heart disease in certain people. It may be given to you for other reasons. Talk with the doctor. Perindopril 1/7 What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have ever had a very bad or life-threatening reaction called angioedema. Signs may be swelling of the hands, face, lips, eyes, tongue, or throat; trouble breathing; trouble swallowing; unusual hoarseness. If you have kidney disease. If you are taking a drug that has aliskiren in it and you also have diabetes or kidney problems. -

Perindopril 4 Mg Tablets Perindopril 8 Mg Tablets Perindopril Tert-Butylamine
Package leaflet: Information for the patient Perindopril 2 mg Tablets Perindopril 4 mg Tablets Perindopril 8 mg Tablets Perindopril tert-butylamine Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor, pharmacist or nurse. This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Perindopril Tablets are and what they are used for 2. What you need to know before you take Perindopril Tablets 3. How to take Perindopril Tablets 4. Possible side effects 5. How to store Perindopril Tablets 6. Contents of the pack and other information 1. What Perindopril Tablets are and what they are used for Perindopril belongs to a group of medicines called ACE inhibitors. These work by widening the blood vessels. This makes it easier for your heart to pump blood through the body. Perindopril Tablets are used to: treat high blood pressure (hypertension). treat heart failure (a condition where the heart is unable to pump enough blood to meet the body’s needs). reduce the risk of cardiac events, such as heart attack, in patients with stable coronary artery disease (a condition where the blood supply to the heart is reduced or blocked) and who have already had a heart attack and/or an operation to improve the blood supply to the heart by widening the vessels that supply it. -

Management of Hypertension Using Olmesartan Alone Or in Combination
Cardiol Ther DOI 10.1007/s40119-017-0087-5 REVIEW Management of Hypertension Using Olmesartan Alone or in Combination Xiaoshen Zhang . Han Zhang . Yuxia Ma . Wenliang Che . Michael R. Hamblin Received: January 17, 2017 Ó The Author(s) 2017. This article is published with open access at Springerlink.com ABSTRACT incidence of hypertension. Concerning the high morbidity rate, setting up an updated Hypertension is one of the most significant and standard for hypertensive patients becomes consistent risk factors for many cardiovascular indispensable. According to the widely accepted diseases. The global prevalence of hypertension standard treatments for hypertension, these has dramatically increased over recent years. four basic principles should be taken into Life-style and genetic factors are generally con- account: low dosage; medication should pro- sidered to be primarily responsible for the vide long term-control; combination therapies are becoming common; personalized treat- ments are a newer approach. In most patients Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/ with hypertension, adequate control of BP can E097F0602FD64106. be achieved with combined therapy. Therefore, antihypertensive agents with complementary X. Zhang Á H. Zhang Á W. Che mechanisms are now recommended. In this Department of Cardiology, Shanghai Tenth Hospital review, we focus on the pharmacology, antihy- of Tongji University, Shanghai 200072, China pertensive efficacy, and adverse events (AEs) of X. Zhang olmesartan medoxomil, either alone or in Tongji University Cancer Institute, Tongji combination with other antihypertensive med- University School of Medicine, Shanghai 200092, ications. In conclusion, olmesartan medoxomil, China is an angiotensin II receptor blocker with an X. -
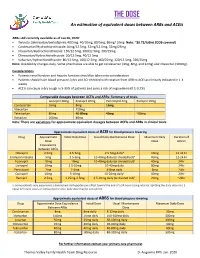
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -

Recommendation to Restrict the Combined Use of Medicines Affecting the Renin-Angiotensin (Ras) System
HPRA page August2014_medbook 12/07/2014 14:23 Page iv Recommendation to RestRict the combined use of medicines affecting the Renin-angiotensin (Ras) system The European Medicines Agency’s Pharmacovigilance Risk individual studies, that the likely benefits from dual Assessment Committee (PRAC) has reviewed the risks of blockade are limited to a reduction in hospital admissions combining different classes of medicines that act on the for heart failure among people with pre-existing heart renin-angiotensin (RAS) system. These medicines, which failure, and highlights some important safety concerns are called RAS-acting agents, belong to three classes: associated with dual therapy. Based on the totality of the 1. Angiotensin-receptor blockers (ARBs) for example evidence, the PRAC has recommended that dual therapy candesartan, telmisartan, valsartan, losartan, should be restricted to limited situations under specialist olmesartan and irbesartan*, supervision after careful consideration of the likely risks and benefits. 2. Angiotensin-converting enzyme inhibitors (ACE inhibitors) for example captopril, enalapril, lisinopril, Following this review, harmonised implementation of these ramipril, perindopril and zofenopril*, recommendations into product information has begun in 3. Direct renin inhibitors such as aliskiren*, which is the order to reflect the available information and adequately only authorised substance in its class. manage the concerns identified with regards to dual RAS blockade therapy and the overall conclusion that dual The PRAC has advised that combining medicines from any blockade of the renin angiotensin system with an ACE two of these classes should not be recommended, and in inhibitor plus an angiotensin receptor blocker (ARB) has particular that patients with diabetic nephropathy should only a limited place in treatment e.g. -

Switching Ace-Inhibitors
Switching Ace-inhibitors http://www.ksdl.kamsc.org.au/dtp/switching_ace_inhibitors.html Change to → Enalapril Quinapril Ramipril Change from ↓ (Once daily dosing) (Once daily dosing) (Once daily dosing) Captopril Captopril 12.5mg daily Enalapril 2.5mg1 Quinapril 2.5mg Ramipril 1.25mg Captopril 25mg daily Enalapril 5mg1 Quinapril 5mg Ramipril 1.25-2.5mg Captopril 50mg daily Enalapril 7.5mg1 Quinapril 10mg Ramipril 2.5-5mg Captopril 100mg daily Enalapril 20mg1 Quinapril 20mg Ramipril 5-10mg2 Captopril 150mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg Fosinopril Fosinopril 5mg daily Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Fosinopril 10mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Fosinopril 20mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Fosinopril 40mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg Lisinopril Lisinopril 5mg daily Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Lisinopril 10mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Lisinopril 20mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Lisinopril 40mg Enalapril 40mg Quinapril 40mg Ramipril 10mg Perindopril Perindopril 2mg daily Enalapril 5-10mg Quinapril 5-10mg Ramipril 2.5mg Perindopril 4mg daily Enalapril 10mg-20mg Quinapril 10mg-20mg Ramipril 5mg Perindopril 8mg daily Enalapril 20-40mg Quinapril 20-40mg Ramipril 10mg Trandolapril Trandolapril 0.5mg d Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Trandolapril 1mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Trandolapril 2mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Trandolapril 4mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg There are few studies comparing equivalent doses of ACE-inhibitors, for specific indications. Therefore, the above recommendations are based on clinical experiences and are not specific for any indication. -

Randomized Trial of Perindopril, Enalapril, Losartan and Telmisartan in Overweight Or Obese Patients with Hypertension
Clin Drug Investig DOI 10.1007/s40261-013-0094-9 ORIGINAL RESEARCH ARTICLE Randomized Trial of Perindopril, Enalapril, Losartan and Telmisartan in Overweight or Obese Patients with Hypertension Serge V. Nedogoda • Alla A. Ledyaeva • Elena V. Chumachok • Vera V. Tsoma • Galina Mazina • Alla S. Salasyuk • Irina N. Barykina Ó Springer International Publishing Switzerland 2013 Abstract other BP, echocardiographic, metabolic and anthropomet- Background and Objectives Obesity exacerbates hyper- ric parameters occurred with all treatments. tension and stimulates the renin–angiotensin–aldosterone Conclusion Full-dose RAAS inhibition, particularly with system (RAAS). Full-dose RAAS inhibition could be a perindopril, effectively reduces BP, improves arterial therapeutic option in overweight or obese patients with structure and regulates cardiovascular risk factors in hypertension. This study compared four RAAS inhibitors overweight or obese patients with hypertension. at full therapeutic doses to determine their effect on blood pressure (BP) and cardiovascular risk factors in these patients. 1 Introduction Methods We conducted a 24-week, single-blind, random- ized, parallel-group study in 120 overweight or obese patients Effective reduction of elevated blood pressure (BP), car- (body mass index C27 kg/m2) with hypertension, aged diovascular prevention and mortality reduction are the 18–60 years. The primary endpoint was the change in mean main goals of antihypertensive therapy [1, 2]. Obesity not 24-h systolic BP and diastolic BP from baseline to study end. only appears to have a substantial pathophysiological effect Central BP, arterial stiffness, and metabolic and cardiac on the haemodynamic changes seen in hypertension but indices were also investigated. Patients were randomly also impairs the response to treatment [3–5]. -

Low-Dose Perindopril and Indapamide Combination Compared with Losartan in the Treatment of Systemic Hypertension: a Randomized, Double-Blind Study
Original ArticlePerindopril and Indapamide versus Losartan in the Treatment of Acta Hypertension Cardiol Sin 2005;21:199-206 Hypertension Low-Dose Perindopril and Indapamide Combination Compared with Losartan in the Treatment of Systemic Hypertension: A Randomized, Double-Blind Study Chien-Hsun Hsia1 and Yu-Deh Su2 Background and Purpose: We compared the efficacy and safety of a low-dose combination of perindopril and indapamide (P/I, 2/0.625 mg) with losartan (50 mg) in Taiwanese patients with mild to moderate essential hypertension. Methods: Fifty patients with mild to moderate essential hypertension were randomized to receive either P/I or losartan once daily for a period of 12 weeks after a 2-week run-in phase. After 8 weeks treatment, if the office blood pressure (BP) was 140/90 mmHg or greater, the dose was doubled. Response and normalization rates (systolic blood pressure [SBP] less than 140 mmHg and diastolic blood pressure [DBP] less than 90 mmHg), extent of BP reduction, and proportion of patients necessitating increase of dose were compared both in full set and per protocol set analyses. Results: The baseline characteristics were comparable between the two patient groups. Normalization rate was significantly higher in the P/I group than in the losartan group (69.2% vs 41.7%) (p < 0.05); so was the response rate (80.8% vs 54.2%) (p < 0.05). Mean sitting SBP and sitting DBP fell by 15.2 ± 10.0 and 9.5 ± 4.7 mmHg, respectively, in the P/I group (p < 0.001) and by 11.6 ± 12.3 and 6.7 ± 7.4 mmHg, respectively, in the losartan group (p < 0.001). -

Controlled Release Nifedipine and Valsartan Combination Therapy In
789 Hypertens Res Vol.29 (2006) No.10 p.789-796 Original Article Controlled Release Nifedipine and Valsartan Combination Therapy in Patients with Essential Hypertension: The Adalat CR and Valsartan Cost-Effectiveness Combination (ADVANCE-Combi) Study Ikuo SAITO1) and Takao SARUTA2), ADVANCE-Combi Study Group* This study was designed to compare the clinical efficacy of two calcium channel blocker–based combina- tion therapies with an angiotensin receptor blocker in Japanese patients with essential hypertension. A 16- week, double-blind, parallel-arm, randomized clinical trial was performed to compare the efficacy and safety of the combination therapy of controlled release nifedipine (nifedipine CR) plus valsartan vs. that of amlo- dipine plus valsartan. The primary endpoint was the target blood pressure achievement rate. Eligible patients were randomly allocated to nifedipine CR–based or amlodipine-based treatment groups. Patients were examined every 4 weeks to determine whether the blood pressure had reached the target level. When the target level was not achieved, the drug regimen was changed; when the target blood pressure was achieved, the same study medication was continued. A total of 505 patients were enrolled in the study (nife- dipine CR group: 245 cases; amlodipine group: 260 cases). After 16 weeks of treatment, blood pressure was significantly reduced in both groups, but to a larger extent in the nifedipine CR group than in the amlodipine group (p<0.01). The target blood pressure achievement rate was also significantly higher in the nifedipine CR group (p<0.001). There was no significant difference in the incidence of drug-related adverse events between the groups. -

PACKAGE LEAFLET: INFORMATION for the PATIENT Perindopril 2Mg, 4Mg & 8Mg Tablets Perindopril Tert-Butylamine
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Perindopril 2mg, 4mg & 8mg Tablets Perindopril Tert-butylamine Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or your pharmacist. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Perindopril is and what it is used for 2. What you need to know before you take Perindopril 3. How to take Perindopril 4. Possible side effects 5. How to store Perindopril 6. Contents of the pack and other information 1. What Perindopril is and what it is used for Perindopril belongs to a group of medicines called ACE Inhibitors. These work by widening the blood vessels. This makes it easier for your heart to pump blood through the body. Perindopril is used to: • Treat high blood pressure (hypertension) • Treat heart failure (a condition where the heart is unable to pump enough blood to meet the body’s needs) • Reduce the risk of cardiac events, such as heart attack, in patients with stable coronary artery disease (a condition where the blood supply to the heart is reduced or blocked) and who have already had a heart attack and/or an operation to improve the blood supply to the heart by widening the vessels that supply it 2.