Лесоведение И Болотоведение Forest and Mire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Moths Count Newsletter 2011
16 Moths Count Newsletter 2011 Half Price Membership Offer Why not become a member of Butterfly Conservation for one Moths Count year at half the usual price? Offer available online from 16 th to 3 1st July 2 011 Ne wsl etter 2011 Membership subscriptions are essential to enable us to The NMRS: Pu tting continue all the important work we do to save threatened moths . By taking advantage of this special half price offer you will not Moths on the Map only get yourself a bargain but will also directly contribute to In the early days of the Moths Count project the the survival of these amazing creatures. Moths Count establishment of a National Moth Recording Scheme (NMRS) Contacts was extremely ambitious, particularly as many vice-counties As a member of Butterfly Conservation didn’t have a computerised dataset; records were stored you will receive the following benefits: General enquiries on a card-index or in some cases even on scraps of paper info @butterfly-conservation.org 01929 400209 in cardboard boxes! Furthermore, 34 vice-counties didn’t I New member welcome pack Richard Fox have an active County Moth Recorder. Fortunately, due to I Our exclusive full-colour magazine Butterfly , three times a year Surveys Manager the enthusiasm and willingness of many individuals these I Membership of your local Butterfly Conservation Branch rfox @butterfly-conservation.org 01626 368385 hurdles were overcome. The moth recording community I Opportunities to take part in monitoring and recording schemes Les Hill rose to the challenge of either volunteering themselves for I Regional newsletters and local events Database Manager the vital role of County Moth Recorder or in assisting in lhill @butterfly-conservation.org 01929 406008 the computerisation of hundreds of thousands of paper To take advantage of this special half price offer join online at Zoë Randle records enabling County Recorders to concentrate on the www.butterfly-conservation.org between 16th and 3 1st July Surveys Officer verification of records. -

Populus Nigra Network
IN SITU CONSERVATION 79 In situ conservation Poplars and biodiversity PeterȱRotachȱ Department of Forest Sciences, Swiss Federal Institute of Technology, Zürich, Switzerland Floodplain forests, the natural habitat of indigenous black poplar (Populus nigra L.), are among the most diverse ecosystems in Europe (Gepp et al. 1985). In Austria, for example, it was estimated that at least 12 000 species of animals and plants regularly inhabit the floodplains of the Danube (Gepp et al. 1985). According to Gerken (1988) more than 1000 species of beetles, most of the indigenous amphibians, 400–500 species of large butterflies (more than one third of all existing species) and between 150 and 200 species of birds occur in different floodplain habitats. Table 1 shows the numbers of invertebrates that have been recorded in the floodplains of the Rhine. Table 1. Number of species of invertebrates in the floodplains of the Rhine, according to Tittizer and Krebs (1996) Order Number of species Mollusca (land snails) >60 Mollusca (water snails and mussels) 30–40 Odonata (dragonflies) 50 Coleoptera (beetles) >1000 Lepidoptera (butterflies) 1000 Arachnida (spiders) >100 Many of the species are highly specialized and depend on alluvial habitats. For example, 29% of the amphibians, 27% of the carabids, 20% of the reptiles and 12% of the dragonfly species in Switzerland occur uniquely or primarily in alluvial habitats (Walter et al. 1998). Undisturbed floodplain ecosystems are not only very rich in species, but also provide a unique or very important habitat for numerous threatened species and thus play a crucial role for species conservation. For insects, mammals, birds, reptiles and amphibians in Switzerland, for example, 17.5% of extinct species, 27% of those that are nearly extinct, 19% of highly threatened species and 11% of threatened species live exclusively, or primarily, in alluvial habitats (Walter et al. -

Contemporary Recordings of Belarusian Folk Biblical and Non-Biblical Etiological Legends in the Comparative-Historical Aspect
https://doi.org/10.7592/FEJF2018.72.boganeva CONTEMPORARY RECORDINGS OF BELARUSIAN FOLK BIBLICAL AND NON-BIBLICAL ETIOLOGICAL LEGENDS IN THE COMPARATIVE-HISTORICAL ASPECT Elena Boganeva Center for Belarusian Culture, Language and Literature Research National Academy of Sciences, Belarus e-mail: [email protected] Abstract: The article considers some rare etiologies in contemporary record- ings – non-biblical cosmogonic and folk biblical anthropogenic etiologies in the comparative and historical aspect. Cosmogonic etiologies are stories about the predetermination of spatial and temporal parameters of the world (texts about the origins of the elements of the earthscape, in particular, mountains; about the agreement made between God and Satan concerning the distribution of as- cendancy over people at the beginning of the world and at the end of it; about the determination of the time of the existence of the world and the change of time); about the structure of the current status of the Universe (the Earth is round, it revolves and is supported by a giant turtle or tortoise); about the primary entity or body, from which the following emerges: the world (out of a child’s body), a part of cultural space – a group of inhabited localities (out of a felled statue), and one of the primary elements – fire (out of a human). Anthropogenic folk biblical etiologies include stories about the origins of sexual relations between the first people (two versions); the birth of children out of different body parts (the head, through the side of the body); and the origins of hair on male bodies. Keywords: anthropogenic etiologies, etiological legend, etiologies of cosmogonic legends, folk Bible, folk biblical etiologies, folk narratives, non-biblical etiologies, vernacular Christianity INTRODUCTION According to its administrative division, there are 6 regions (oblasts) and 118 districts in Belarus. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Review-Chronicle
REVIEW-CHRONICLE OF THE HUMAN RIGHTS VIOLATIONS IN BELARUS IN 1999 2 REVIEW-CHRONICLE OF THE HUMAN RIGHTS VIOLATIONS IN BELARUS IN 1999 INTRODUCTION: GENERAL CONCLUSIONS The year of 1999 was the last year of Alexander Lukashenka’s original mandate. In 1994 having used the machinery of democratic procedure he was elected president of the Republic of Belarus for five years term. But in 1996 A.Lukashenka conducted illegal, non-free and unfair referendum and by it prolonged his mandate to seven years. Constitutional Court’s judges and deputies of the Supreme Soviet that resisted to A.Lukashenka’s dictatorial intentions were dismissed. Thus provisions of the Constitution of the Republic of Belarus were broken. Attempt to conduct presidential elections done by the legitimate Supreme Soviet of the 13th convocation was supported by the most influential opposition parties and movements. But Belarusan authorities did their best to prevent opposition from succeeding in presidential elections and subjected people involved in election campaign to different kinds of repressions. Regime didn’t balk at anything in the struggle with its opponents. Detentions and arrests, persecutions of its organisers and participants, warnings, penalties and imprisonment followed every opposition-organised action… Yet the year of 1999 became a year of mass actions of protest of Belarusan people against a union with Russia imposed by the authorities to the people. In 1999 the OSCE Advisory and Monitoring Group in Belarus made an attempt to arrange talks between Belarusan authorities and opposition. This year will go down to history as a year when some of prominent politicians and fighters against the regime disappeared, when unprecedented number of criminal proceedings against opposition leaders and participants of mass actions of protest was instituted.. -

Database of Irish Lepidoptera. 1 - Macrohabitats, Microsites and Traits of Noctuidae and Butterflies
Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Irish Wildlife Manuals No. 35 Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Ken G.M. Bond and Tom Gittings Department of Zoology, Ecology and Plant Science University College Cork Citation: Bond, K.G.M. and Gittings, T. (2008) Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies. Irish Wildlife Manual s, No. 35. National Parks and Wildlife Service, Department of the Environment, Heritage and Local Government, Dublin, Ireland. Cover photo: Merveille du Jour ( Dichonia aprilina ) © Veronica French Irish Wildlife Manuals Series Editors: F. Marnell & N. Kingston © National Parks and Wildlife Service 2008 ISSN 1393 – 6670 Database of Irish Lepidoptera ____________________________ CONTENTS CONTENTS ........................................................................................................................................................1 ACKNOWLEDGEMENTS ....................................................................................................................................1 INTRODUCTION ................................................................................................................................................2 The concept of the database.....................................................................................................................2 The structure of the database...................................................................................................................2 -
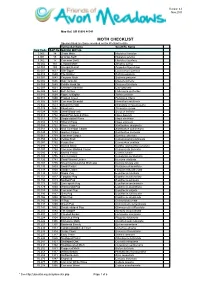
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

The Meaning of the Centers for Pluralism for Belarus 39
36 Stojan Obradoviæ and Eric Chenoweth 37 Looking at the nearly ten years of NIJ’s existence, STINA is proud of its The Meaning of the Centers for achievements. Its work was not spectacular – we did not aim for splashy sto- ries. But it was significant and important. NIJ was alone in covering some of Pluralism for Belarus the key transition stories of this period, whether it was the prevalence of cor- ruption, the political uses of ethnic conflict and nationalism, the misuses of by Vincuk Viaèorka and Siarhiej Mackieviè privatization, or the ignored stories of civil society. Most importantly, the NIJ covered the development of democracy – and lack thereof – in the postcom- Vincuk Viaèorka is chairman of Belarus’ leading opposition party, the Belarus munist region. We brought to light the parties, individuals, and processes that Popular Front (BPF), which was founded in 1988. He is also the founder and for- many media ignored, but which proved to be among the most important mer chairman of the Belarus Center for Pluralism, the Civil Society Center- actors in the decade’s key democratic events. Supolnasc, a Center for Pluralism begun in 1996. Siarhiej Mackieviè is Supolnasc’s Today, due to sudden financial difficulties, the NIJ has had to suspend current chairman. He was chief of the National Headquarters of the non-partisan service temporarily. Nevertheless, it is planning further development and electoral mobilization campaign “Vybirai” (Choose) in 2001 and is vice chairman growth in the future. The goal of the Network of Independent Journalists is of the Assembly of Pro-Democratic NGOs. -

Scottish Macro-Moth List, 2015
Notes on the Scottish Macro-moth List, 2015 This list aims to include every species of macro-moth reliably recorded in Scotland, with an assessment of its Scottish status, as guidance for observers contributing to the National Moth Recording Scheme (NMRS). It updates and amends the previous lists of 2009, 2011, 2012 & 2014. The requirement for inclusion on this checklist is a minimum of one record that is beyond reasonable doubt. Plausible but unproven species are relegated to an appendix, awaiting confirmation or further records. Unlikely species and known errors are omitted altogether, even if published records exist. Note that inclusion in the Scottish Invertebrate Records Index (SIRI) does not imply credibility. At one time or another, virtually every macro-moth on the British list has been reported from Scotland. Many of these claims are almost certainly misidentifications or other errors, including name confusion. However, because the County Moth Recorder (CMR) has the final say, dubious Scottish records for some unlikely species appear in the NMRS dataset. A modern complication involves the unwitting transportation of moths inside the traps of visiting lepidopterists. Then on the first night of their stay they record a species never seen before or afterwards by the local observers. Various such instances are known or suspected, including three for my own vice-county of Banffshire. Surprising species found in visitors’ traps the first time they are used here should always be regarded with caution. Clerical slips – the wrong scientific name scribbled in a notebook – have long caused confusion. An even greater modern problem involves errors when computerising the data. -

Assessment of the Agriculture and Rural Development Sectors in the Eastern Partnership Countries
The European Union’s Neighbourhood Programme Assessment of the Agriculture and Rural Development Sectors in the Eastern Partnership countries Republic of Belarus Funded by the European Union Implemented by FAO FAO Regional Office for Europe and Central Asia (REU) Benczur utca 34. H-1068 Budapest Hungary Tel: +36 1 4612000 Fax: +36 1 3517029 E-mail: [email protected] FAO Project No. GCP/RER/041/EC EU Project No. ENPI 2012/298-262 Start date: 16th July, 2012 End date: 31st December, 2012 This publication has been produced with the assistance of the European Union. The contents of this publication are the sole responsibility of the FAO and can in no way be taken to reflect the views of the European Union. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. List of abbreviations ARD Agricultural and Rural development BERAS Baltic Ecological Recycling Agriculture and Society BSU Belarusian State University BYR Belarusian Ruble (National currency of Belarus) CAP Common Agricultural -
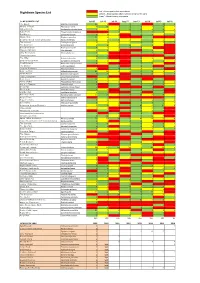
Highdown Species List 2008 and 2009 and 2010 and 2011 and 2012 and 2013 and 2014 and 2015 and 2016.Xlsx
'red' = those species that were absent Highdown Species List 'yellow' = those species who's numbers remained the same 'green' = those showing an increase CORE SURVEY LIST Jul‐09 Jul‐10 Jul‐11 Aug‐12 Jun‐13 Jul‐14 Jul‐15 Jul‐16 The Snout Hypena proboscidalis 1142322 Mother of Pearl Pleuroptya ruralis 911334242 Willow Beauty Peribatodes rhomboidaria 76368491 Ruby Tiger Phragmatobia fuliginosa 212232 Buff Ermine Spilosoma luteum 862 2913 Muslin Moth Diaphora mendica 12 6 2 1 Broad-bordered Yellow Underwing Noctua fimbriata 52621311 The Coronet Craniophora ligustri 43114725 The Sycamore Acronicta aceris 2323232 Burnished Brass Diachrysia chrysitis 632332 Chinese Character Cilix glaucata 131 21 Grey Pine Carpet Thera obeliscata 74 333 Carcina quercana 23 1 The Miller Acronicta leporine 68 642 Swallow-tailed Moth Ourapteryx sambucaria 951 6243 Small Emerald Hemistola chrysoprasaria 81 232 The Drinker Euthrix potatoria 1122 Swallow Prominent Pheosia tremula 4 34212 Rosy Footman Miltochrista miniata 4118224946 White Ermine Spilosoma lubricipeda 791 3623 True Lover's Knot Lycophotia porphyrea 1 Knot Grass Acronicta rumicis 127352 Angle Shades Phlogophora meticulosa 84114121 Nut-tree Tussock Colocasia coryli 4 4 3341 Brown-tail Euproctis chrysorrhoea 72 24 Blood-vein Timandra comae 72 1112 Slender Pug Eupithecia tenuiata 24 5 1 Brimstone Moth Opisthograptis luteolata 11 19 2 7 12 6 6 4 Purple Thorn Selenia tetralunaria 1 2 Peppered Moth Biston betularia 75126253 Buff Arches Habrosyne pyritoides 532 4464 Setaceous Hebrew Character Xestia c‐nigrum -

Checklist of Cumbrian Lepidoptera (2000)
A Checklist of the Butterflies and larger Moths of Cumbria Edited by Bill Kydd and Stephen Hewitt A Checklist of the Butterflies and larger Moths of Cumbria Edited by Bill Kydd and Stephen Hewitt February 2000 Tullie House Museum and Art Gallery Castle Street Carlisle © Carlisle City Council 2000 The Cumbria Biological Records Database at Tullie House Museum Tullie House Museum maintains a database of wildlife sightings from across the county of Cumbria. Some 140,000 records from Cumbria are stored on computer, backed up by large collections of voucher and reference specimens. The Museum aims to record and monitor the status of wildlife in Cumbria. The information is used to increase the knowledge of the wildlife of the county and to inform decisions affecting the wildlife and countryside of Cumbria. Tullie House welcomes information and records concerning the wildlife of Cumbria. Provisional distribution atlases are available for the following groups: Mammals in Cumbria 1999 Butterflies in Cumbria 2000 Dragonflies in Cumbria 1994 Grasshoppers and Crickets in Cumbria 1993 Ladybirds in Cumbria 1998 Reptiles in Cumbria 1992 Amphibians in Cumbria 1995 Please send your wildlife sightings to the Keeper of Natural Sciences, Tullie House Museum, Castle Street, Carlisle CA3 8TP. Tel: 01228 534781 ext. 248. Fax: 01228 810249. E-mail: [email protected] Introduction The purpose of this checklist is to assist local moth recorders in assessing their own records and captures. We hope that it will enable recorders to identify unusual and important records as they occur and further stimulate the study of moths in Cumbria. Bill Kydd acted as County Lepidoptera Recorder for Cumbria Naturalists’ Union from 1979 to 1997.