Policy Implications of the Computed Tomography (CT) Scanner (Part 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening for Postmenopausal Osteoporosis
This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. AHRQ is the lead Federal agency charged with supporting research designed to improve the quality of health care, reduce its cost, address patient safety and medical errors, and broaden access to essential services. AHRQ sponsors and conducts research that provides evidence-based information on health care outcomes; quality; and cost, use, and access. The information helps health care decisionmakers— patients and clinicians, health system leaders, and policymakers—make more informed decisions and improve the quality of health care services. Systematic Evidence Review Number 17 Screening for Postmenopausal Osteoporosis Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 2101 East Jefferson Street Rockville, MD 20852 http://www.ahrq.gov Contract No. 290-97-0018 Task Order No. 2 Technical Support of the U.S. Preventive Services Task Force Prepared by: Oregon Health Sciences University Evidence-based Practice Center, Portland, Oregon Heidi D. Nelson, MD, MPH Mark Helfand, MD, MS September 2002 Preface The Agency for Healthcare Research and Quality (AHRQ) sponsors the development of Systematic Evidence Reviews (SERs) through its Evidence-based Practice Program. With ∗ guidance from the third U.S. Preventive Services Task Force (USPSTF) and input from Federal partners and primary care specialty societies, two Evidence-based Practice Centers—one at the Oregon Health Sciences University and the other at Research Triangle Institute-University of North Carolina—systematically review the evidence of the effectiveness of a wide range of clinical preventive services, including screening, counseling, immunizations, and chemoprevention, in the primary care setting. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

A-Scan Echoencephalography in Measurement of the Cerebral Ventricles
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.31.3.245 on 1 June 1968. Downloaded from J. Neurol. Neurosurg. Psychiat., 1968, 31, 245-249 A-scan echoencephalography in measurement of the cerebral ventricles ANAND G. GARG AND ALEX. R. TAYLOR From the Department ofNeurological Surgery, Royal Victoria Hospital, Belfast, Northern Ireland The first attempts at ultrasonic visualization of the METHOD cerebral ventricles were made by Dussik (1948), The ventricular measurements obtained at echoen- Ballantine, Ludwig, Bolt, and Hueter (1950), and cephalography were compared with the x-ray measure- Hueter and Bolt (1951), using the transmission ments made at pneumoencephalography. method. The possible use of the pulse-echo method ANATOMICAL CONSIDERATIONS The third and lateral (echoencephalography) for the diagnosis of hy- ventricles are supratentorial structures. The third ventricle lies between the two thalmi, communicating drocephalus was suggested by Leksell (1956). Later in front with the lateral ventricles through the inter- Kikuchi, Uchida, Tanaka, and Wagai (1957) and de ventricular foramina and behind with the aqueduct of Vlieger and Ridder (1959) recorded echoes from the the midbrain. The septum lucidum and the third ventricle walls of the lateral ventricles. According to Gordon lie in the central plane of the brain. Protected by copyright. (1959), and de Vlieger and Ridder (1959), the width The lateral ventricle is a C-shaped cavity lying within of the third ventricle can also be measured. ter the cerebral hemisphere. It consists of a central body and Braak, Crezde, Grandia, and de Vleger (1961) used three horns-anterior, posterior, and temporal-running pneumoencephalography to study the origin of into the frontal, occipital, and temporal lobes respec- ventricular echoes. -

Evicore Pediatric Head Imaging V1.0.2019 Eff 02.15.19
CLINICAL GUIDELINES Pediatric Head Imaging Policy Version 1.0.2019 Effective February 15, 2019 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Imaging Guidelines V1.0.2019 Pediatric Head Imaging Procedure Codes Associated with Pediatric Head Imaging 3 PEDHD-1: General Guidelines 5 PEDHD-2: Specialized Imaging Techniques 10 PEDHD-3: Pediatric Headache 13 PEDHD-4: Pediatric Head and Face Trauma 15 PEDHD-5: Sinusitis and Allergic Rhinitis 18 PEDHD-6: Epilepsy and Other Seizure Disorders 22 PEDHD-7: Macrocephaly, Microcephaly, and Hydrocephalus 26 PEDHD-8: Craniosynostosis 30 PEDHD-9: Chiari and Skull Base Malformations 32 PEDHD-10: Intracranial Aneurysms -
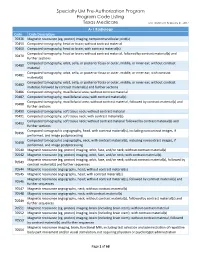
Specialty UM Pre-Authorization Program Code Listing
Specialty UM Pre-Authorization Program Program Code Listing Texas Medicare Last Updated: February 21, 2017 A-1 Radiology Code Code Description 70336 Magnetic resonance (eg, proton) imaging, temporomandibular joint(s) 70450 Computed tomography, head or brain; without contrast material 70460 Computed tomography, head or brain; with contrast material(s) Computed tomography, head or brain; without contrast material, followed by contrast material(s) and 70470 further sections Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; without contrast 70480 material Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; with contrast 70481 material(s) Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; without contrast 70482 material, followed by contrast material(s) and further sections 70486 Computed tomography, maxillofacial area; without contrast material 70487 Computed tomography, maxillofacial area; with contrast material(s) Computed tomography, maxillofacial area; without contrast material, followed by contrast material(s) and 70488 further sections 70490 Computed tomography, soft tissue neck; without contrast material 70491 Computed tomography, soft tissue neck; with contrast material(s) Computed tomography, soft tissue neck; without contrast material followed by contrast material(s) and 70492 further sections Computed tomographic angiography, head, with contrast material(s), including noncontrast images, if 70496 performed, and image postprocessing -

Clinical Experience with Automatic Midline Echoencephalography: Cooperative Study of Three Neurosurgical Clinics'
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.38.3.272 on 1 March 1975. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1975, 38, 272-278 Clinical experience with automatic midline echoencephalography: cooperative study of three neurosurgical clinics' M. KLINGER, E. KAZNER,2 TH. GRUMME, V. AMTENBRINK, G. GRAEF, K. H. HARTMANN, H. HOPMAN, W. MEESE, AND B. VOGEL From the Neurosurgical University Clinics, Berlin, Erlangen, and Miinchen, GFR SYNOPSIS Computerized midline echoencephalography was developed in order to make the determination of the midline more objective. In a group study involving the neurosurgical clinics in Berlin, Erlangen, and Munich, a total of 1 889 patients with various intracranial diseases was examined by this method. An exact analysis of the results obtained is presented: 18 % were un- satisfactory. A-scan echoencephalography has become this would limit the number of investigators Protected by copyright. widely used in recent years and is now an indis- considerably. White (1972), on the other hand, pensable tool in the diagnosis of intracranial goes so far as to say that he does not consider lesions (Grossman, 1966; Pia and Geletneky, such a test to be of clinical value if it depends 1968; Schiefer et al., 1968). However, the upon the presence of a neurological specialist. literature contains many reports on the difficul- ties this method presents, especially for the METHOD beginner (McKinney, 1964; Jefferson and Hill, 1966, 1968; Kramer, 1968; White, 1967, 1970, In an effort to eliminate as much as possible the 1972; White and Hanna, 1974). Experienced factor 'experience' in the determination of the mid- line echo and to provide scientific objectivity, there investigators have emphasized for years that has been no lack of attempts to standardize this prolonged practice is necessary before echo- examination, especially on the North American encephalography can yield truly reliable and continent. -

OUTPATIENT FACILITY NATIONWIDE CHARGES by CPT/HCPCS CODE V3.27 (January - December 2020) PAGE 1 of 177
TABLE F. — OUTPATIENT FACILITY NATIONWIDE CHARGES BY CPT/HCPCS CODE v3.27 (January - December 2020) PAGE 1 of 177 CPT/ Multiple Surgery Status/ Usage Charge HCPCS Description Reduction Charge Indicator 1 Methodology 2 Code Applies 10004 FNA BX W/O IMG GDN EA ADDL e Blank $1,893.87 v3.25 10005 FNA BX W/US GDN 1ST LES Blank Blank $2,886.01 APC 10006 FNA BX W/US GDN EA ADDL e Blank $2,778.06 v3.25 10007 FNA BX W/FLUOR GDN 1ST LES Blank Blank $2,886.01 APC 10008 FNA BX W/FLUOR GDN EA ADDL e Blank $2,778.06 v3.25 10009 FNA BX W/CT GDN 1ST LES Blank Blank $2,886.01 APC 10010 FNA BX W/CT GDN EA ADDL e Blank $2,778.06 v3.25 10011 FNA BX W/MR GDN 1ST LES Blank Blank $2,886.01 APC 10012 FNA BX W/MR GDN EA ADDL e Blank $2,778.06 v3.25 10021 FINE NEEDLE ASPIRATION W/O IMAGING GUIDANCE Blank Blank $1,852.76 APC 10030 IMAGE-GUIDED CATHETER FLUID COLLECTION DRAINAGE Blank Blank $3,258.68 APC 10035 PERQ SFT TISS LOC DEVICE PLMT 1ST LES W/GDNCE Blank Blank $3,258.68 APC 10036 PERQ SFT TISS LOC DEVICE PLMT ADD LES W/GDNCE e Blank $2,298.09 FAIR Health 10040 ACNE SURGERY Blank Blank $654.01 APC 10060 INCISION & DRAINAGE ABSCESS SIMPLE/SINGLE Blank Blank $654.01 APC 10061 INCISION & DRAINAGE ABSCESS COMPLICATED/MULTIPLE Blank Blank $1,852.76 APC 10080 INCISION & DRAINAGE PILONIDAL CYST SIMPLE Blank Blank $3,258.68 APC 10081 INCISION & DRAINAGE PILONIDAL CYST COMPLICATED Blank Blank $3,258.68 APC 10120 INCISION & REMOVAL FOREIGN BODY SUBQ TISS SIMPLE Blank Blank $1,852.76 APC 10121 INCISION & REMOVAL FOREIGN BODY SUBQ TISS COMPL Blank Blank $8,354.68 APC -

Products of Ambulatory Care 2004 Procedure Codes
Products of Ambulatory Care 2004 Procedure Codes KeyTech CPT CPT Description AUD 92506 Evaluation of speech, language, voice, communication, auditory processing, and/or aural rehabilitation status AUD 92531 Spontaneous nystagmus, including gaze AUD 92532 Positional nystagmus test AUD 92533 Caloric vestibular test, each irrigation (binaural, bithermal stimulation constitutes four tests) AUD 92534 Optokinetic nystagmus test AUD 92543 Caloric vestibular test, each irrigation (binaural, bithermal stimulation constitutes four tests), with recording AUD 92546 Sinusoidal vertical axis rotational testing AUD 92548 Computerized dynamic posturography AUD 92551 Screening test, pure tone, air only AUD 92552 Pure tone audiometry (threshold); air only AUD 92553 Pure tone audiometry (threshold); air and bone AUD 92555 Speech audiometry threshold; AUD 92556 Speech audiometry threshold; with speech recognition AUD 92557 Comprehensive audiometry threshold evaluation and speech recognition (92553 and 92556 combined) AUD 92559 Audiometric testing of groups AUD 92560 Bekesy audiometry; screening AUD 92561 Bekesy audiometry; diagnostic AUD 92562 Loudness balance test, alternate binaural or monaural AUD 92563 Tone decay test AUD 92564 Short increment sensitivity index (SISI) AUD 92565 Stenger test, pure tone AUD 92567 Tympanometry (impedance testing) AUD 92568 Acoustic reflex testing AUD 92569 Acoustic reflex decay test AUD 92571 Filtered speech test AUD 92572 Staggered spondaic word test AUD 92573 Lombard test AUD 92575 Sensorineural acuity level test AUD -

Masshealth Transmittal Letter IDTF-6 July 2008 TO
Commonwealth of Massachusetts Executive Office of Health and Human Services Office of Medicaid 600 Washington Street Boston, MA 02111 www.mass.gov/masshealth MassHealth Transmittal Letter IDTF-6 July 2008 TO: Independent Diagnostic Testing Facility Providers Participating in MassHealth FROM: Tom Dehner, Medicaid Director RE: Independent Diagnostic Testing Facility Manual (2008 HCPCS) This letter transmits revisions to the service codes Subchapter 6 of in the Independent Diagnostic Testing Facility Manual. The Centers for Medicare & Medicaid Services (CMS) have revised the Healthcare Common Procedure Coding System (HCPCS) for 2008. The revised Subchapter 6 is effective for dates of service on or after July 1, 2008. An IDTF provider may request prior authorization (PA) for any medically necessary service reimbursable under the federal Medicaid Act in accordance with 130 CMR 450.144, 42 U.S.C.1396d(a), and 42 U.S.C. 1396d(r)(5) for a MassHealth Standard or CommonHealth member younger than 21 years of age, even if it is not designated as covered or payable in the Independent Diagnostic Testing Facility Manual. If you wish to obtain a fee schedule, you may download the Division of Health Care Finance and Policy regulations at no cost at www.mass.gov/dhcfp. You may also purchase a paper copy of Division of Health Care Finance and Policy regulations from either the Massachusetts State Bookstore or from the Division of Health Care Finance and Policy (see addresses and telephone numbers below). You must contact them first to find out the price of the paper copy of the publication. The regulation titles for this provider are 114.3 CMR 16: Surgery and Related Anesthesia, 114.3 CMR 17: Medicine and 114.3 CMR 18: Radiology. -

Two-Dimensional Echoencephalography with Electronic Sector Scanning Clinical Experiences with a New Method
Journal ofNeurology, Neurosurgery, and Psychiatry, 1972, 35, 912-918 Two-dimensional echoencephalography with electronic sector scanning Clinical experiences with a new method H. A. C. KAMPHUISEN, J. C. SOMER, AND W. A. OOSTERBAAN Department of Clinical Neurophysiology, University of Utrecht, and the Institute of Medical Physics T.N.O., Utrecht, The Netherlands SUMMARY A new form of ultrasound diagnostic possibility is presented (the electroscan). The basic principles of this two dimensional method are described. Special attention is given to the probe consisting of an array of 21 elements of piezo-electric material. The results of this method in four patients are discussed (meningioma, arteriovenous aneurysm, subdural haematoma, and a baby with hydrocephalus). The baby with hydrocephalus showed diagnostic problems which could be under- stood from the pneumoencephalographic findings. The electroscan method seems to offer good possibilities for the diagnosis of brain lesions, ifthese have a consistency different from that of normal brain tissue. Conventional echoencephalography is based on difficulty from an echo answering from a real the pulse-echo method, in which a transducer layer, as would be possible in a two-dimensional transmits short ultrasound pulses, the echoes of scanning system. which are received back during the intervals. These echoes are visualized as vertical excursions on an oscilloscope-screen, while the position on ELECTRONIC SCANNING PRINCIPLE the screen is a measure for the distance at which (Somer, 1968) the reflecting structure is situated. The direction Two-dimensional scanning for obtaining cross- of the emitted ultrasound pulses is always per- sectional pictures can be performed both mech- pendicular to the surface of the probe and thus it anically and electronically. -

Martin's Point Radiology & Cardiology Prior Authorization
Martin's Point Radiology & Cardiology Prior Authorization Categories CPT® CPT Description 3D Imaging 76376 3D Rendering W/O Postprocessing 3D Imaging 76377 3D Rendering W Postprocessing CT 75571 Computed tomography, heart, without contrast material, with quantitative evaluation of coronary calcium Computed tomography, heart, with contrast material, for evaluation of cardiac structure and morphology (including 3d image CT 75572 postprocessing, assessment of cardiac function, and evaluation of venous structures, if performed) Computed tomography, heart, with contrast material, for evaluation of cardiac structure and morphology in the setting of congenital CT 75573 heart disease (including 3d image postprocessing, assessment of lv cardiac function, rv structure and function and evaluation of venous structures, if performed) Computed tomographic angiography, heart, coronary arteries and bypass grafts (when present), with contrast material, including 3d CT 75574 image postprocessing (including evaluation of cardiac structure and morphology, assessment of cardiac function, and evaluation of venous structures, if performed) Cardiac Services 93303 Transthoracic echocardiography for congenital cardiac anomalies; complete Cardiac Services 93304 Transthoracic echocardiography for congenital cardiac anomalies; follow-up or limited study Echocardiography, transthoracic, real-time with image documentation (2d), includes m-mode recording, when performed, complete, Cardiac Services 93306 with spectral doppler echocardiography, and with color flow doppler -

Medicare National Coverage Determinations Manual Chapter 1, Part 4 (Sections 200 – 310.1) Coverage Determinations
Medicare National Coverage Determinations Manual Chapter 1, Part 4 (Sections 200 – 310.1) Coverage Determinations Table of Contents (Rev. 142, 02-03-12) Transmittals for Chapter 1, Part 4 200 - Pharmacology 200.1 - Nesiritide for Treatment of Heart Failure Patients (Effective March 2, 2006) 200.2 - Nebulized Beta Adrenergic Agonist Therapy for Lung Diseases - (Effective September 10, 2007) 210 - Prevention 210.1 - Prostate Cancer Screening Tests 210.2 - Screening Pap Smears and Pelvic Examinations for Early Detection of Cervical or Vaginal Cancer 210.3 – Colorectal Cancer Screening Tests 210.4 – Smoking and Tobacco-Use Cessation Counseling (Effective March 22, 2005) 210.4.1 – Counseling to Prevent Tobacco Use (Effective August 25, 2010) 210.5 - Diabetes Screening Tests (Effective January 1, 2005) 210.7 – Screening for the Human Immunodeficiency Virus (HIV) Infection (Effective December 8, 2009) 210.8 – Screening and Behavioral Counseling Interventions in Primary Care to Reduce Alcohol Misuse (Effective October 14, 2011) 210.9 – Screening for Depression in Adults (Effective October 14, 2011) 210.11 - Intensive Behavioral Therapy for Cardiovascular Disease 210.12 – Intensive Behavioral Therapy for Obesity 220 - Radiology 220.1 - Computed Tomography 220.2 - Magnetic Resonance Imaging (MRI) (Various Effective Dates Below) 220.2.1 - Magnetic Resonance Spectroscopy 220.3 - Magnetic Resonance Angiography 220.4 - Mammograms 220.5 - Ultrasound Diagnostic Procedures (Effective May 22, 2007) 220.6 – Positron Emission Tomography (PET) Scans (Effective