Technical Assessment of Ultrasonic Cerebral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Introduction to Neuroimaging
Introduction to Neuroimaging Aaron S. Field, MD, PhD Assistant Professor of Radiology Neuroradiology Section University of Wisconsin–Madison Updated 7/17/07 Neuroimaging Modalities Radiography (X-Ray) Magnetic Resonance (MR) Fluoroscopy (guided procedures) • MR Angiography/Venography (MRA/MRV) • Angiography • Diffusion and Diffusion Tensor • Diagnostic MR • Interventional • Perfusion MR • Myelography • MR Spectroscopy (MRS) Ultrasound (US) • Functional MR (fMRI) • Gray-Scale Nuclear Medicine ―Duplex‖ • Color Doppler • Single Photon Emission Computed Tomography (SPECT) Computed Tomography (CT) • Positron Emission Tomography • CT Angiography (CTA) (PET) • Perfusion CT • CT Myelography Radiography (X-Ray) Radiography (X-Ray) Primarily used for spine: • Trauma • Degenerative Dz • Post-op Fluoroscopy (Real-Time X-Ray) Fluoro-guided procedures: • Angiography • Myelography Fluoroscopy (Real-Time X-Ray) Fluoroscopy (Real-Time X-Ray) Digital Subtraction Angiography Fluoroscopy (Real-Time X-Ray) Digital Subtraction Angiography Digital Subtraction Angiography Indications: • Aneurysms, vascular malformations and fistulae • Vessel stenosis, thrombosis, dissection, pseudoaneurysm • Stenting, embolization, thrombolysis (mechanical and pharmacologic) Advantages: • Ability to intervene • Time-resolved blood flow dynamics (arterial, capillary, venous phases) • High spatial and temporal resolution Disadvantages: • Invasive, risk of vascular injury and stroke • Iodinated contrast and ionizing radiation Fluoroscopy (Real-Time X-Ray) Myelography Lumbar or -

Neurological Critical Care: the Evolution of Cerebrovascular Critical Care Cherylee W
50TH ANNIVERSARY ARTICLE Neurological Critical Care: The Evolution of Cerebrovascular Critical Care Cherylee W. J. Chang, MD, FCCM, KEY WORDS: acute ischemic stroke; cerebrovascular disease; critical FACP, FNCS1 care medicine; history; intracerebral hemorrhage; neurocritical care; Jose Javier Provencio, MD, FCCM, subarachnoid hemorrhage FNCS2 Shreyansh Shah, MD1 n 1970, when 29 physicians first met in Los Angeles, California, to found the Society of Critical Care Medicine (SCCM), there was little to offer for the acute management of a patient suffering from an acute cerebrovascular Icondition except supportive care. Stroke patients were not often found in the ICU. Poliomyelitis, and its associated neuromuscular respiratory failure, cre- ated a natural intersection of neurology with critical care; such was not the case for stroke patients. Early textbooks describe that the primary decision in the emergency department was to ascertain whether a patient could swallow. If so, the patient was discharged with the advice that nothing could be done for the stroke. If unable to swallow, a nasogastric tube was inserted and then the patient was discharged with the same advice. In the 50 intervening years, many advances in stroke care have been made. Now, acute cerebrovascular patients are not infrequent admissions to an ICU for neurologic monitoring, observa- tion, and aggressive therapy (Fig. 1). HISTORY Over 50 years ago, stroke, previously called “apoplexy” which means “struck down with violence” or “to strike suddenly,” was a clinical diagnosis that was confirmed by autopsy as a disease of the CNS of vascular origin (1). In the 1960s, approximately 25% of stroke patients died within 24 hours and nearly half died within 2 to 3 weeks. -

Intracranial Cerebrovascular Evaluations: Transcranial Doppler
VASCULAR TECHNOLOGY PROFESSIONAL PERFORMANCE GUIDELINES Intracranial Cerebrovascular Evaluations: Transcranial Doppler Ultrasound and Transcranial Color Duplex Imaging This Guideline was prepared by the Professional Guidelines Subcommittee of the Society for Vascular Ultrasound (SVU) as a template to aid the vascular technologist/sonographer and other interested parties. It implies a consensus of those substantially concerned with its scope and provisions. The guidelines contain recommendations only and should not be used as a sole basis to make medical practice decisions. This SVU Guideline may be revised or withdrawn at any time. The procedures of SVU require that action be taken to reaffirm, revise, or withdraw this Guideline no later than three years from the date of publication. Suggestions for improvement of this guideline are welcome and should be sent to the Executive Director of the Society for Vascular Ultrasound. No part of this guideline may be reproduced in any form, in an electronic retrieval system or otherwise, without the prior written permission of the publisher. Sponsored and published by: Society for Vascular Ultrasound 4601 Presidents Drive, Suite 260 Lanham, MD 20706-4831 Tel.: 301-459-7550 Fax: 301-459-5651 E-mail: [email protected] Internet: www.svunet.org Copyright © by the Society for Vascular Ultrasound, 2019 ALL RIGHTS RESERVED. PRINTED IN THE UNITED STATES OF AMERICA. VASCULAR PROFESSIONAL PERFOMANCE GUIDELINE Updated January 2019 Intracranial Cerebrovascular Evaluation s: TCD and TCDI 01/2019 PURPOSE Transcranial Doppler ultrasound (TCD) and transcranial color duplex imaging (TCDI) studies measure blood flow velocities and direction within portions of the intracranial arteries. TCD and TCDI evaluate the anterior and posterior circulation territories to assess and manage patients with cerebrovascular disease. -

Screening for Postmenopausal Osteoporosis
This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. AHRQ is the lead Federal agency charged with supporting research designed to improve the quality of health care, reduce its cost, address patient safety and medical errors, and broaden access to essential services. AHRQ sponsors and conducts research that provides evidence-based information on health care outcomes; quality; and cost, use, and access. The information helps health care decisionmakers— patients and clinicians, health system leaders, and policymakers—make more informed decisions and improve the quality of health care services. Systematic Evidence Review Number 17 Screening for Postmenopausal Osteoporosis Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 2101 East Jefferson Street Rockville, MD 20852 http://www.ahrq.gov Contract No. 290-97-0018 Task Order No. 2 Technical Support of the U.S. Preventive Services Task Force Prepared by: Oregon Health Sciences University Evidence-based Practice Center, Portland, Oregon Heidi D. Nelson, MD, MPH Mark Helfand, MD, MS September 2002 Preface The Agency for Healthcare Research and Quality (AHRQ) sponsors the development of Systematic Evidence Reviews (SERs) through its Evidence-based Practice Program. With ∗ guidance from the third U.S. Preventive Services Task Force (USPSTF) and input from Federal partners and primary care specialty societies, two Evidence-based Practice Centers—one at the Oregon Health Sciences University and the other at Research Triangle Institute-University of North Carolina—systematically review the evidence of the effectiveness of a wide range of clinical preventive services, including screening, counseling, immunizations, and chemoprevention, in the primary care setting. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

2Nd Quarter 2001 Medicare Part a Bulletin
In This Issue... From the Intermediary Medical Director Medical Review Progressive Corrective Action ......................................................................... 3 General Information Medical Review Process Revision to Medical Record Requests ................................................ 5 General Coverage New CLIA Waived Tests ............................................................................................................. 8 Outpatient Hospital Services Correction to the Outpatient Services Fee Schedule ................................................................. 9 Skilled Nursing Facility Services Fee Schedule and Consolidated Billing for Skilled Nursing Facility (SNF) Services ............. 12 Fraud and Abuse Justice Recovers Record $1.5 Billion in Fraud Payments - Highest Ever for One Year Period ........................................................................................... 20 Bulletin Medical Policies Use of the American Medical Association’s (AMA’s) Current Procedural Terminology (CPT) Codes on Contractors’ Web Sites ................................................................................. 21 Outpatient Prospective Payment System January 2001 Update: Coding Information for Hospital Outpatient Prospective Payment System (OPPS) ......................................................................................................................... 93 he Medicare A Bulletin Providers Will Be Asked to Register Tshould be shared with all to Receive Medicare Bulletins and health care -

A-Scan Echoencephalography in Measurement of the Cerebral Ventricles
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.31.3.245 on 1 June 1968. Downloaded from J. Neurol. Neurosurg. Psychiat., 1968, 31, 245-249 A-scan echoencephalography in measurement of the cerebral ventricles ANAND G. GARG AND ALEX. R. TAYLOR From the Department ofNeurological Surgery, Royal Victoria Hospital, Belfast, Northern Ireland The first attempts at ultrasonic visualization of the METHOD cerebral ventricles were made by Dussik (1948), The ventricular measurements obtained at echoen- Ballantine, Ludwig, Bolt, and Hueter (1950), and cephalography were compared with the x-ray measure- Hueter and Bolt (1951), using the transmission ments made at pneumoencephalography. method. The possible use of the pulse-echo method ANATOMICAL CONSIDERATIONS The third and lateral (echoencephalography) for the diagnosis of hy- ventricles are supratentorial structures. The third ventricle lies between the two thalmi, communicating drocephalus was suggested by Leksell (1956). Later in front with the lateral ventricles through the inter- Kikuchi, Uchida, Tanaka, and Wagai (1957) and de ventricular foramina and behind with the aqueduct of Vlieger and Ridder (1959) recorded echoes from the the midbrain. The septum lucidum and the third ventricle walls of the lateral ventricles. According to Gordon lie in the central plane of the brain. Protected by copyright. (1959), and de Vlieger and Ridder (1959), the width The lateral ventricle is a C-shaped cavity lying within of the third ventricle can also be measured. ter the cerebral hemisphere. It consists of a central body and Braak, Crezde, Grandia, and de Vleger (1961) used three horns-anterior, posterior, and temporal-running pneumoencephalography to study the origin of into the frontal, occipital, and temporal lobes respec- ventricular echoes. -

Evicore Pediatric Head Imaging V1.0.2019 Eff 02.15.19
CLINICAL GUIDELINES Pediatric Head Imaging Policy Version 1.0.2019 Effective February 15, 2019 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Imaging Guidelines V1.0.2019 Pediatric Head Imaging Procedure Codes Associated with Pediatric Head Imaging 3 PEDHD-1: General Guidelines 5 PEDHD-2: Specialized Imaging Techniques 10 PEDHD-3: Pediatric Headache 13 PEDHD-4: Pediatric Head and Face Trauma 15 PEDHD-5: Sinusitis and Allergic Rhinitis 18 PEDHD-6: Epilepsy and Other Seizure Disorders 22 PEDHD-7: Macrocephaly, Microcephaly, and Hydrocephalus 26 PEDHD-8: Craniosynostosis 30 PEDHD-9: Chiari and Skull Base Malformations 32 PEDHD-10: Intracranial Aneurysms -

June-July 2000 Part a Bulletin
In This Issue... Disclosure of Itemized Statement Providers Must Furnish an Itemmized Statement when Requested in Writing by the Beneficiary .................................................................................................... 6 Prospective Payment System The Outpatient Code Editor Software Has Been Modified in Preparation for the Implementation of Outpatient Prospective Payment System ........................... 8 Reclassification of Certain Urban Hospitals Certain Urban Hospitals in the State of Florida May Be Permitted to Be Reclassified as Rural Hospitals................................................................................ 13 ulletin Final Medical Review Policies 33216, 53850, 70541, 82108, 83735, 87621, 93303, 94010, 95004, A0320, J0207, J2430, J2792, J3240, J7190, and J9999 .......................................... 15 B Payment of Skilled Nursing Facility Claims Involving a Terminating Medicare+Choice Plan Payment of Skilled Nursing Facility Care for Beneficiaries Involuntarily Disenrolling from M+C plans Who Have Not Met the 3-Day Stay Requirement ........................................................................................... 67 Features From the Medical Director 3 Administrative 4 lease share the Medicare A roviders PBulletin with appropriate General Information 5 members of your organization. Outpatient Prospective Payment System 7 Routing Suggestions: General Coverage 12 o Medicare Manager Hospital Services 13 o Reimbursement Director Local and Focused Medical Policies 15 o Chief Financial -
Brain Spect with Tl-201 Ddc
BRAIN SPECT WITH TL-201 DDC INIS-mf —11361 BRAIN SPECT WITH TL-201 DDC BRAIN SPECT WITH TL-201 DDC ACADEMISCH PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit van Amsterdam, op gezag van de Rector Magnificus Prof, dr S.K. Thoden van Velzen, in het openbaar te verdedigen in de Aula der Universiteit (Oude Lutherse Kerk, ingang Singel 411, hoek Spui), op donderdag 21 april 1988 te 13.30 uur door Johan Frederik de Brume geboren te 's Gravenhage AMSTERDAM 1988 Promotor : Prof, dr J.B. van der Schoot Copromotor : Dr E.A. van Royen To my parents Joey, Joost and Duco The publication of this thesis was financially supported by: The Netherlands Heart Foundation CIL, BV, Mallinckrodt General Electric Medical Systems Printed in the Netherlands Rodopi B.V., Amsterdam ISBN: 90-900-2127-2 Contents Chapter 1: Introduction 1 Chapter 2: Current methods in neuroimaging and cerebral blood flow measurements 5 2.1. Angiography and digital subtraction angiography 5 2.2. Duplex sonography 7 2.3. Technetium-99m pertechnetate brainscintigraphy 8 2.4. Regional cerebral blood flow measurements with Xenon-133 8 2.5. Computed tomography and Xenon-enhanced computed tomography 9 2.6. Nuclear magnetic resonance imaging 13 2.7. Positron emission tomography 15 2.8. Single-photon emission computed tomography 16 2.9. Regional cerebral blood flow imaging and blood- brain barrier 25 2.10. Cerebral blood flow tracers for SPECT 27 2.11. Possible applications of SPECT brain studies 32 Chapter 3: Functional brain imaging with 1-123 amphetamine First experience in the Netherlands 53 Chapter 4: Thallium-201 diethyldithiocarbamate 69 4.1. -
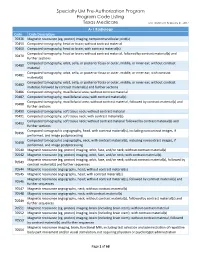
Specialty UM Pre-Authorization Program Code Listing
Specialty UM Pre-Authorization Program Program Code Listing Texas Medicare Last Updated: February 21, 2017 A-1 Radiology Code Code Description 70336 Magnetic resonance (eg, proton) imaging, temporomandibular joint(s) 70450 Computed tomography, head or brain; without contrast material 70460 Computed tomography, head or brain; with contrast material(s) Computed tomography, head or brain; without contrast material, followed by contrast material(s) and 70470 further sections Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; without contrast 70480 material Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; with contrast 70481 material(s) Computed tomography, orbit, sella, or posterior fossa or outer, middle, or inner ear; without contrast 70482 material, followed by contrast material(s) and further sections 70486 Computed tomography, maxillofacial area; without contrast material 70487 Computed tomography, maxillofacial area; with contrast material(s) Computed tomography, maxillofacial area; without contrast material, followed by contrast material(s) and 70488 further sections 70490 Computed tomography, soft tissue neck; without contrast material 70491 Computed tomography, soft tissue neck; with contrast material(s) Computed tomography, soft tissue neck; without contrast material followed by contrast material(s) and 70492 further sections Computed tomographic angiography, head, with contrast material(s), including noncontrast images, if 70496 performed, and image postprocessing -

Clinical Experience with Automatic Midline Echoencephalography: Cooperative Study of Three Neurosurgical Clinics'
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.38.3.272 on 1 March 1975. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1975, 38, 272-278 Clinical experience with automatic midline echoencephalography: cooperative study of three neurosurgical clinics' M. KLINGER, E. KAZNER,2 TH. GRUMME, V. AMTENBRINK, G. GRAEF, K. H. HARTMANN, H. HOPMAN, W. MEESE, AND B. VOGEL From the Neurosurgical University Clinics, Berlin, Erlangen, and Miinchen, GFR SYNOPSIS Computerized midline echoencephalography was developed in order to make the determination of the midline more objective. In a group study involving the neurosurgical clinics in Berlin, Erlangen, and Munich, a total of 1 889 patients with various intracranial diseases was examined by this method. An exact analysis of the results obtained is presented: 18 % were un- satisfactory. A-scan echoencephalography has become this would limit the number of investigators Protected by copyright. widely used in recent years and is now an indis- considerably. White (1972), on the other hand, pensable tool in the diagnosis of intracranial goes so far as to say that he does not consider lesions (Grossman, 1966; Pia and Geletneky, such a test to be of clinical value if it depends 1968; Schiefer et al., 1968). However, the upon the presence of a neurological specialist. literature contains many reports on the difficul- ties this method presents, especially for the METHOD beginner (McKinney, 1964; Jefferson and Hill, 1966, 1968; Kramer, 1968; White, 1967, 1970, In an effort to eliminate as much as possible the 1972; White and Hanna, 1974). Experienced factor 'experience' in the determination of the mid- line echo and to provide scientific objectivity, there investigators have emphasized for years that has been no lack of attempts to standardize this prolonged practice is necessary before echo- examination, especially on the North American encephalography can yield truly reliable and continent.