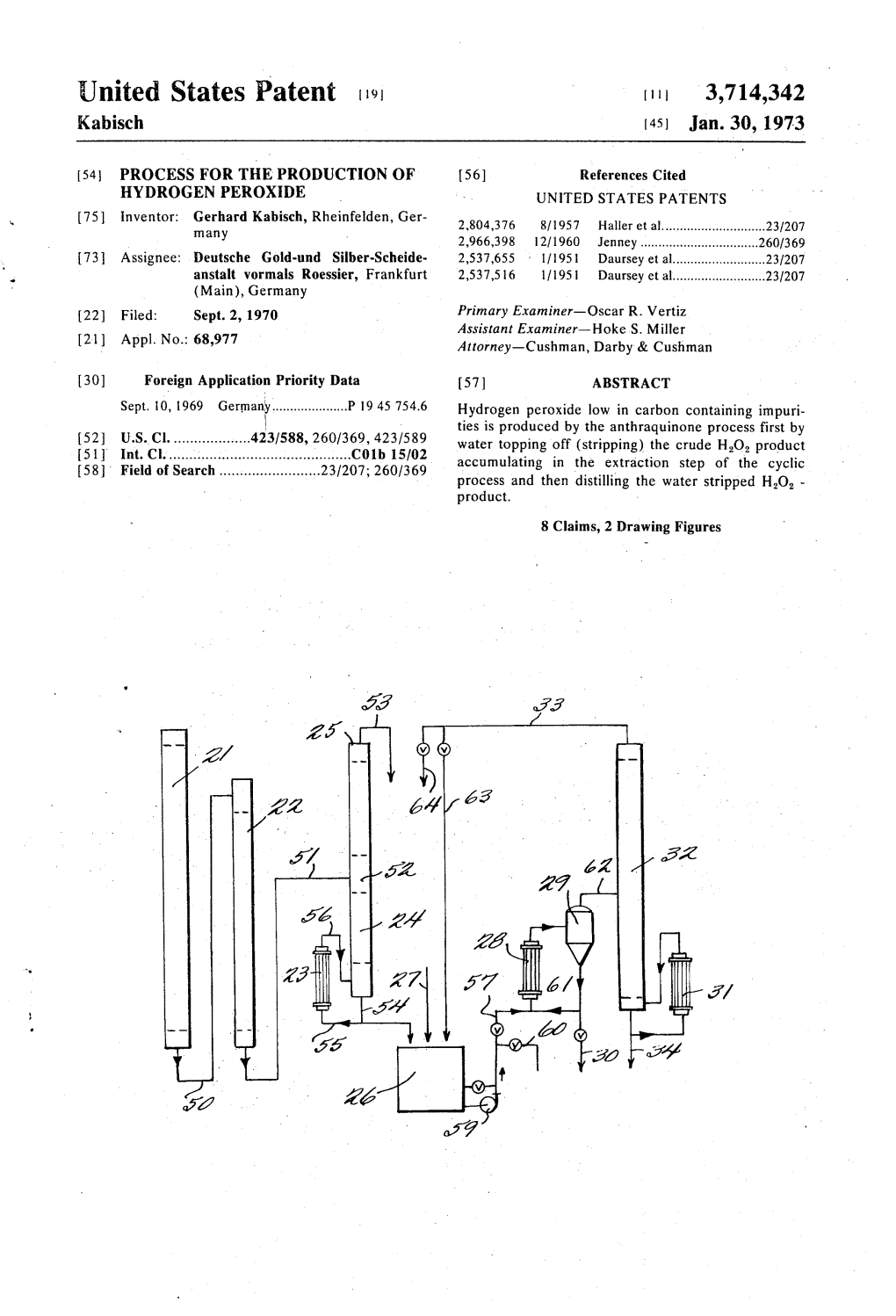
United States Patent (19) 1 3,714,342 Kabisch (45) Jan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Challenge Solution Application Note · Multi N/C® 2100S / 3100 Analysis
Application Note · multi N/C® 2100S / 3100 Analysis of TOC/TN in Product Quality Monitoring of Hydrogen Peroxide Introduction Hydrogen peroxide is used in many processes, for example, as an environmentally friendly means of bleaching pulp, paper, and textiles; as a disinfectant in the food industry or in water purification; or as an oxidation agent in chemical synthesis. According to the different fields of application, products of various H2O2 concentrations and purity grades are available. The industrial production of hydrogen peroxide is done via the anthraquinone process, followed by cleanup Challenge steps, such as fractional distillation. To guarantee product quality, H O is monitored for elemental impurities, for Reproducible and precise 2 2 example, for heavy metals or for TOC concentration. This application note focusses determination of TOC and TN on the determination of TOC and TN in undiluted H O . The TOC concentration contents in the lower ppm range 2 2 in H O samples can vary in a wide range from approx. 1 to 500 mg/l depending in highly concentrated H O 2 2 2 2 on the manner of product stabilization and grade of cleanup. TN is a parameter matrices (up to 70% H O ). 2 2 optionally monitored for process control but not, however, typically specified for final products. Since H2O2 is a strong oxidant and sensitive to decomposition, the Solution way in which the sample is handled and the robustness of the materials used inside the TOC/TN analyzer are crucial for the overall stability of the analytical Fully automated and method. simultaneous TOC/TN The measurements were performed on a multi N/C® 2100S and a measurement using catalytic multi N/C® 3100. -

United States Patent Office Patented Mar
3,432,267 United States Patent Office Patented Mar. 11, 1969 2 cordingly, the term "degradation products' as used herein 3,432,267 does not apply to tetrahydro derivatives of the anthraqui REGENERATION OF ANTHRAQUINONE WORK none working compound. NG SOLUTION USED IN THE PRODUCTION OF HYDROGEN PEROXDE In U.S. Patent 2,739,875, issued to Jerome W. Sprauer Nathan Dean Lee, Lambertville, and Nelson Nor on Mar. 27, 1956, there is described a process for treating man Schwartz, Trenton, N.J., assignors to FMC Cor an anthraquinone working solution containing degradation poration, New York, N.Y., a corporation of Delaware products whereby the solution can be regenerated to re No Drawing. Filled May 26, 1967, Ser. No. 641,458 store its hydrogen peroxide synthesizing capacity. In ac U.S. C. 23-207 8 Clains cordance with this process, the anthraquinone working Int, C. C01b. 15/02 solution is heated in the presence of either activated alu O mina or activated magnesia, thereby regenerating its hy drogen peroxide synthesizing capacity. ABSTRACT OF THE DISCLOSURE While this process has been found to be useful and A degraded anthraquinone working solution (made up effective in regenerating some working solutions during of an anthraquinone working compound dissolved in one 5 initial use, after repeated cyclic hydrogenation and oxida or more solvents) used in the production of hydrogen tion of an anthraquinone working solution, it develops a peroxide is regenerated by treating it with ozone, extract resistance to being regenerated by activated alumina or ing the resulting solution with an aqueous caustic solution, magnesia. -

Reduction of O to H O Using Small Polycyclic Molecules
Journal Name † Reduction of O2 to H2O2 using Small Polycyclic Molecules Kristal Lopez,a and Michael N Groves∗a Hydrogen peroxide is an environmentally friendly oxidizing agent that is important in several in- dustries. It is currently produced industrially via the anthrahydroquinone (AHQ) process where O2 reacts with a functionalised version of anthrahydroquinone to produce H2O2 and anthraquinone. In the previously published DFT pathway for this process the transition of the OOH· radical across the partially dehydrogenated AHQ catalyst was not explored. In this paper, we will use DFT to explore this step and show that there is a deep potential energy minimum that inhibits the OOH· from being fully reduced. We then examine other similar sized polycyclic molecules with two OH-groups on the same side that could serve as alternative catalysts without this issue. In this analysis, we identify phenanthraquinone as a possible alternative and present the pathway for this candidate to produce H2O2 as well as its regeneration with H2. 1 Introduction limit the fire hazard given the explosive nature of H2/O2 mixtures Hydrogen peroxide (H2O2) is used in many industries including as well as the inclusion of methanol as a solvent 13. Supercriti- as a bleaching agent for paper production 1–5 and water treat- cal CO2 has also been explored as a solvent to replace methanol, 6–8 ment . Worldwide production exceeds 5.5 million metric tons however, the reaction temperature plays a significant role. In a 3 in 2015 and it continues to climb . It is also used in several study by Landon et al. -

Hydrogen Peroxide Colorimetric Activity Kit
DetectX® Hydrogen Peroxide Colorimetric Activity Kit 2 Plate Kit Catalog Number K034-H1 Species Independent Sample Types Validated: Fresh Urine, Buffers and TCM Please read this insert completely prior to using the product. For research use only. Not for use in diagnostic procedures. www.ArborAssays.com K034-H1 WEB 191127 TABLE OF CONTENTS Background 3 Assay Principle 4 Related Products 4 Supplied Components 4 Storage Instructions 4 Other Materials Required 5 Precautions 5 Sample Types and Preparation 5 Reagent Preparation 6 Assay Protocol 7 Calculation of Results 7 Typical Data 8 Validation Data Sensitivity, Linearity, etc. 8-10 Warranty & Contact Information 11 Plate Layout Sheet 12 ® 2 EXPECT ASSAY ARTISTRY™ K034-H1 WEB 191127 BACKGROUND Hydrogen peroxide was first described in 1818 by Louis Jacques Thénard. Today, industrially, hydrogen peroxide is manufactured almost exclusively by the autoxidation of a 2-alkyl-9,10-dihydroxyanthracene to the corresponding 2-alkyl anthraquinone in the Riedl-Pfleiderer or anthraquinone process. - In biological systems incomplete reduction of O2 during respiration produces superoxide anion (O2 ·), which is spontaneously or enzymatically dismutated by superoxide dismutase to H2O2. Many cells produce low - levels of O2 · and H2O2 in response to a variety of extracellular stimuli, such as cytokines (TGF-ß1, TNF-a, and various interleukins), peptide growth factors (PDGF; EGF, VEGF, bFGF, and insulin), the agonists of heterotrimeric G protein–coupled receptors (GPCR) such as angiotensin II, thrombin, lysophosphatidic acid, 1 sphingosine 1-phosphate, histamine, and bradykinin, and by shear stress . The addition of exogenous H2O2 or the intracellular production in response to receptor stimulation affects the function of various proteins, including protein kinases, protein phosphatases, transcription factors, phospholipases, ion channels, and 2 2+ G proteins. -

Regeneration Method Development for Used Alumina in the Hydrogen Peroxide Process
DF Regeneration method development for used alumina in the hydrogen peroxide process Master’s thesis in Innovative and Sustainable Chemical Engineering Gandhi Rajamani Balasubramanian Department of Chemistry and Chemical Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2020 Master’s thesis 2020 Regeneration method development for used alumina in the hydrogen peroxide process Gandhi Rajamani Balasubramanian DF Department of Chemistry and Chemical Engineering Division of Chemical Reaction Engineering Chalmers University of Technology Gothenburg, Sweden 2020 Regeneration method development for used alumina in the hydrogen peroxide process Gandhi Rajamani Balasubramanian © Gandhi Rajamani Balasubramanian, 2020. Supervisor: Cecilia Andersson, Nouryon Derek Creaser, Chemical Engineering Examiner: Derek Creaser Master’s Thesis 2020 Department of Chemistry and Chemical Engineering Division of Chemical Reaction Engineering Chalmers University of Technology SE-412 96 Gothenburg Telephone +46 31 772 1000 Cover: Photo by the author. Upper Figure: Soxhlet Regenerated alumina for dif- ferent solvents, Lower figure: Solvent Extracted liquid after Soxhlet extraction Typeset in LATEX, template by David Frisk Printed by Chalmers Reproservice Gothenburg, Sweden 2020 iv Regeneration method development for used alumina in the hydrogen peroxide process Gandhi Rajamani Balasubramanian Department of Chemistry and Chemical Engineering Chalmers University of Technology Abstract Hydrogen peroxide is one of the majorly used bleaching chemicals in the pulp and paper industries worldwide. Among the various production process of hydrogen peroxide, Anthraquinone process (also known as autoxidation or AO process) is the widely used industrial route. The use of alumina as a catalyst is used in the AO process for the recovery of active quinones from the inactive anthraquinones [Chen, 2008]. However, this repeated usage of alumina results in loosing its ability to regenerate these inactive anthraquinones and hence limited by its short usage of time. -

Process for Preparation of Hydrogen Peroxide
Europaisches Patentamt J) European Patent Office ® Publication number: 0 286 610 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 88850082.4 ® Int.CI.4: C 01 B 15/023 Date of filing: 09.03.88 Priority: 27.03.87 SE 8701293 @ Applicant: Eka Nobel Aktiebolag S-44501Surte (SE) Date of publication of application: 12.10.88 Bulletin 88/41 @ Inventor: Bengtsson, Erik Alvar Asbacken 45 Designated Contracting States: S-44500Surte (SE) AT BE DE FR GB IT SE Andersson, Ulf, Mikael Bangegatan 2B S-41504G6teborg (SE) @ Representative: Schold, Zaid c/o Nobel Industries Sweden AB Patent Department Box 11554 S-1 00 61 Stockholm (SE) @ Process for preparation of hydrogen peroxide. (57) A process for the production of hydrogen peroxide according to the anthraquinone process. According to the process certain alkyl substituted caprolactames are used as solvents, and particularly as solvents for anthrahydroquinones. The substituted caprolactames, which for example can be hexyl caprolactam and octyl caprolactam, give a very good solubility for anthrahydroquinones and also for anthraquinones. The compounds can be used as the sole solvent at the production of hydrogen peroxide or in combination with conventionally used solvents such CM as hydrocarbons. < O to CO 00 CM Q_ LLJ Bundesdruckerei Berlin 0 286 610 , Description A process for the production of hydrogen peroxide The present invention relates to the production of hydrogen peroxide according to the per se well known anthraquinone process. More particularly the invention relates to the production of hydrogen peroxide 5 according to the anthraquinone process using particular solvents which give very good solubility for anthrahydroquinones and also for anthraquinones. -

Hydrogen Peroxide Fluorescent Detection Kit Manual; Document: #F18-9131-9-A; Effective: 03/04/2015; Supersedes: None; 4 Pages
Hydrogen Peroxide Fluorescent Detection Kit Kit #9131, 2 plates RESEARCH USE ONLY 1. Introduction 2. Kit Contents Hydrogen peroxide was first described in 1818 by Louis Jacques Thé- • 2 black half-area polystyrene 96-well microtiter plates #268 nard. Today, hydrogen peroxide is industrially manufactured almost • 1 vial of Hydrogen Peroxide Standard (220 µL) #6603: Hydro- exclusively by the autoxidation of a 2-alkyl-9,10-dihydroxyanthracene gen Peroxide at 100 µM in a special stabilizing solution (Section to the corresponding 2-alkyl anthraquinone in the Riedl-Pfleiderer or 12). anthraquinone process. • 1 bottle of Assay Buffer Concentrate (25 mL) #6605: A 5X buf- fer concentrate containing detergents and stabilizers (Section 10). In biological systems, incomplete reduction of O2 during respira- - • 1 vial of Fluorescent H O Detection Substrate (5 mL) #6606: A tion produces superoxide anion (O2 ·), which is spontaneously or 2 2 solution of the substrate in a special stabilizing buffer. enzymatically dismutated by superoxide dismutase to H2O2. Many - • 1 vial of Horseradish Peroxidase Concentrate (60 µL) #6607: A cells produce low levels of O2 · and H2O2 in response to a variety of extracellular stimuli, such as cytokines (TGF-ß1, TNF-α, and various 100X concentrated solution of HRP in a special stabilizing solu- interleukins), peptide growth factors (PDGF; EGF, VEGF, bFGF, tion (Section 11). and insulin), the agonists of heterotrimeric G protein–coupled recep- tors (GPCR) such as angiotensin II, thrombin, lysophosphatidic acid, 3. Required Materials sphingosine 1-phosphate, histamine, and bradykinin, and by shear • Repeater pipet with disposable tips capable of accurately dispens- 1 stress . The addition of exogenous H2O2 or the intracellular produc- ing 25 µL. -

The Anthraquinone Process
COVER STORY: H₂O₂ BACKGROUND How H₂O₂ is produced: The anthraquinone process Healthy and durable: In The industrial production of hydrogen peroxide began in the town of Weißenstein in the Austrian state of the food industry, PET bottles are sterilized Carinthia. This is where the Österreichische Chemische Werke company operated the world’s first hydrogen with hydrogen peroxide peroxide factory using electrolysis. Today this production plant is part of Evonik. The Weißenstein process before being filled made it possible to produce hydrogen peroxide on an industrial scale for the first time. Today this plant uses the autoxidation process, as do almost all the other hydrogen peroxide factories in the world. This process was developed by Georg Pfleiderer and Hans-Joachim Riedl at IG Farben in Ludwigshafen between 1935 and 1945, and since then it has been continuously refined. The process is based on the cyclical reduction and oxidation of an alkylated anthraquinone. The first step, hydrogenation, takes place in a reactor full of a solution of the anthraquinone (the “working solution”). “In the reactor, in the presence of a palladium catalyst hydrogen combines with the reaction carrier, a quinone derivative, to form a hydroquinone,” explains Dr. Jürgen Glenneberg, Head of Process Engineering at the Active Oxygens Business Line. The catalyst is then completely filtered out of the working solution. In the second step, the oxidation stage, huge compressors pump air into a bubble reactor that is full of the working solution. When the hydroquinone in the organic phase comes into contact with the oxygen in the air, it oxidizes spontaneously back into quinone, forming hydrogen peroxide in the process. -

Building and Identifying Highly Active Oxygenated Groups in Carbon
ARTICLE https://doi.org/10.1038/s41467-020-15782-z OPEN Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2 ✉ Gao-Feng Han 1, Feng Li 1 , Wei Zou2, Mohammadreza Karamad3, Jong-Pil Jeon1, Seong-Wook Kim1, ✉ Seok-Jin Kim 1, Yunfei Bu 4, Zhengping Fu2,5, Yalin Lu 2,5, Samira Siahrostami 6 & ✉ Jong-Beom Baek 1 1234567890():,; The one-step electrochemical synthesis of H2O2 is an on-site method that reduces depen- dence on the energy-intensive anthraquinone process. Oxidized carbon materials have pro- ven to be promising catalysts due to their low cost and facile synthetic procedures. However, the nature of the active sites is still controversial, and direct experimental evidence is pre- sently lacking. Here, we activate a carbon material with dangling edge sites and then decorate them with targeted functional groups. We show that quinone-enriched samples exhibit high selectivity and activity with a H2O2 yield ratio of up to 97.8 % at 0.75 V vs. RHE. Using density functional theory calculations, we identify the activity trends of different possible quinone functional groups in the edge and basal plane of the carbon nanostructure and determine the most active motif. Our findings provide guidelines for designing carbon-based catalysts, which have simultaneous high selectivity and activity for H2O2 synthesis. 1 School of Energy and Chemical Engineering/Center for Dimension-Controllable Organic Frameworks, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, South Korea. 2 CAS Key Laboratory of Materials for Energy Conversion, Department of Materials Science and Engineering, University of Science and Technology of China (USTC), 230026 Hefei, P. -

Hydrogenation of 2-Ethylanthraquinone with Pd Supported on Hollow Ceramic Microsphere Catalyst: an Experimental and Kinetic Study Anjali A
NCNE-2020 Special Issue J. Indian Chem. Soc., Vol. 97, July 2020, pp. 1033-1037 Hydrogenation of 2-ethylanthraquinone with Pd supported on hollow ceramic microsphere catalyst: An experimental and kinetic study Anjali A. Inglea, Shahid Z. Ansarib, Diwakar Z. Shendea, Kailas L. Wasewara and Aniruddha B. Pandit*b aChemical Engineering Department, Vidyasagar National Institute of Technology Nagpur, Nagpur-440 010, Maharashtra, India bDepartment of Chemical Engineering, Institute of Chemical Technology, Mumbai-40019, India E-mail: [email protected] Manuscript received online 10 April 2020, accepted 08 June 2020 The catalytic hydrogenation of 2-ethylanthraquinone in the anthraquinone process of production of hydrogen peroxide gain- ing more interest to meet environmental demands of hydrogen peroxide as green oxidant. Noble metal palladium becomes a new category of catalyst due to their significant applicability in the catalytic hydrogenation reactions. Herein, wet impregna- tion method was used for synthesis of palladium supported on hollow ceramic microsphere catalysts. The palladium supported on hollow ceramic microsphere exhibits high catalytic selectivity, activity and stability in the liquid phase hydrogenation of 2- ethylanthraquinone. The reaction obeyed zero and first order kinetics with 2-ethylanthraquinone and hydrogen respectively. The average value of reaction rate constant observed was 2 mol L–1 h–1 and 6 L h–1 respectively. Highest yield of hydrogen peroxide obtained was 96.5% with a less consumption of 2-ethylanthraquinone. Keywords: Hollow ceramic microsphere, palladium catalyst, 2-ethylanthraquinone hydrogenation. Introduction cess improvement6,7. Although extensive efforts have been made in the development of the hydrogenation step in the Hydrogen peroxide (H2O2) is one of the promising oxi- dant in various industries, particularly in the chemical and AQ process, there are still problems in reaction kinetic and environmental protection industries1. -

General Technoeconomic Analysis for Electrochemical Coproduction Coupling Carbon Dioxide Reduction with Organic Oxidation
ARTICLE https://doi.org/10.1038/s41467-019-12744-y OPEN General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation Jonggeol Na 1,7, Bora Seo1, Jeongnam Kim1,2, Chan Woo Lee 3, Hyunjoo Lee1,4, Yun Jeong Hwang 1,4,5, Byoung Koun Min 1,6, Dong Ki Lee 1, Hyung-Suk Oh 1,4* & Ung Lee 1,4,6* 1234567890():,; Electrochemical processes coupling carbon dioxide reduction reactions with organic oxidation reactions are promising techniques for producing clean chemicals and utilizing renewable energy. However, assessments of the economics of the coupling technology remain question- able due to diverse product combinations and significant process design variability. Here, we report a technoeconomic analysis of electrochemical carbon dioxide reduction reaction–organic oxidation reaction coproduction via conceptual process design and thereby propose potential economic combinations. We first develop a fully automated process synthesis framework to guide process simulations, which are then employed to predict the levelized costs of chemicals. We then identify the global sensitivity of current density, Faraday efficiency, and overpotential across 295 electrochemical coproduction processes to both understand and predict the levelized costs of chemicals at various technology levels. The analysis highlights the promise that coupling the carbon dioxide reduction reaction with the value-added organic oxidation reaction can secure significant economic feasibility. 1 Clean Energy Research Center, Korea Institute of Science and Technology (KIST), 02792 Seoul, Republic of Korea. 2 School of Chemical and Biological Engineering, Seoul National University, Gwanak-ro 1, Gwanak-gu, 08826 Seoul, Republic of Korea. 3 Department of Chemistry, Kookmin University, 02707 Seoul, Republic of Korea. -

Extraction of Byproducts of Hydrogen Peroxide Working Solution Using Solvent
"Science Stays True Here" Biological and Chemical Research, Volume 2014, 34-39 | Science Signpost Publishing Extraction of Byproducts of Hydrogen Peroxide Working Solution Using Solvent M Arshad Majeed, Suneela Sardar and S R Malik NFC Institute of Engineering and Fertilizer Research, Faisalabad. Received: September 03, 2014 / Accepted: September 27, 2014 / Published: November 25, 2014 Abstract: Working solution comprising anthraquinone and its derivatives, used for preparation of hydrogen peroxide, leads to the formation of byproducts. These byproducts contain highly complex mixture of degradation products, which cannot take active part in the production of hydrogen peroxide, and cause higher viscosity and density of working solution. The low viscosity is important for mechanical reasons in cycling of the working solution through the equipment and density must be substantially different from the density of the water hydrogen peroxide solution resulting from water extraction of the hydrogen peroxide from the organic phase, in order to facilitate the extraction. The degradation products must be extracted from the working solution to prevent deteriorating the crude hydrogen peroxide color, smell, dissolved organic compounds and increase in density and viscosity of the working solution. A decrease in surface tension of the working solution promotes the formation of an emulsion during extraction degradation products decrease the activity and life time of the hydrogenation catalyst. Regeneration of the working solution for bringing it back to the good health a solvent Tetra Butyl Urea (TBU) is added in degraded working solution in a different proportion. Keywords: Regeneration, Anthraquinone, Tetra Butyl Urea, Degradation. 1. Introduction Widely used method for production of hydrogen peroxide is auto-oxidation process in which 2-ethyl-anthraquinone (EAQ) is dissolved in a carrier solvent such as Tri-Octyl Phosphate(TOP) and Tri methyl Benzene(TMB).