Development of a Risk Assessment Tool for Mercury in Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MANAGEMENT DIRECTION STATEMENT November 2003
Skeena Region MANAGEMENT DIRECTION STATEMENT November 2003 for Sutherland River Provincial Park and Sutherland River Protected Area Ministry of Water, Land and Air Protection Environmental Stewardship Division The Vanderhoof Land and Resource Management Plan and the Lakes District Land and Resource Management Plan provided direction for this management direction statement. Table of Contents Page Introduction Purpose of the Management Direction Statement ...........................................................................1 Context.............................................................................................................................................1 Provincial Park and Protected Area Attributes Conservation....................................................................................................................................2 Recreation and Tourism...................................................................................................................4 Commercial Business Opportunities................................................................................................4 Cultural Heritage..............................................................................................................................4 Significance in the Protected Areas System ....................................................................................4 Land Uses, Tenures and Interests Access ..............................................................................................................................................6 -

Indian Reserves Allotted for Fishing Purposes in British Columbia, 1849-1925
Indian Reserves Allotted for Fishing Purposes in British Columbia, 1849-1925 Douglas C. Harris This table accompanies Landing Native Fisheries: Indian Reserves and Fishing Rights in British Columbia, 1849-1925, by Douglas C. Harris (Vancouver: UBC Press, 2008). It provides additional and supporting detail for the Indian reserves that were specifically allotted for fishing purposes. The table is organized geographically and by First Nations language group or regional affiliation. It begins with the reserves allotted to the Coast Salish, and then moves inland, following the Fraser River, to the reserves allotted to the Nlha7kapmx, Stl’alt’imc, Secwepmec, and Tsilqot’in. It then moves east into the Columbia River drainage basin to include the reserves allotted to the Okanagan and Ktunaxa. The table then returns to the coast and works north from the Nuu-chah-nulth reserves on the west coast of Vancouver Island, to the reserves of the Kwakwaka’wkaw, Oweekeno, Heiltsuk, Nuxalk, Haisla, Tsimshian, Haida, and Nisga’a. It then follows the Skeena River inland, listing the reserves allotted to the Gitxsan and Wet’suwet’en, and then the Dakleh reserves of the upper Fraser and Skeena rivers. Finally, it lists the Sekani and Tahltan reserves in the north. With the exception of the reserves allotted under Treaty 8 in north eastern British Columbia, the table lists every reserve allotted for fishing purposes in British Columbia, the First Nation to which the reserve was allotted, the date of the allotment, the person responsible for allotting the reserve, and an extract from the document creating or confirming the reserve that included a reference to the fishery. -

2009 Annual Report
Annual Report bc treaty commission / www.bctreaty.net 2009 treaty commission annual report 2009 | B treaty commission annual report 2009 | 1 2 4 8 12 16 32 letter from our the year looking table about the treaty the chief purpose in review forward reports commission commissioner treaty commission annual report 2009 | 2 Letter from the Chief Commissioner One response to this concern was the development of the Common Table, a group comprising representatives of Canada, BC and more than 60 First Nations that met in mid-2008. This Table was tasked with identifying obstacles, addressing barriers and promoting the speedy conclusion of fair and viable agreements based on the recognition and reconciliation of aboriginal title and rights. The participants were expected to take the outcomes of the Most of the accomplishments in my life were made work to their respective decision-makers for consideration possible by the gifts of wisdom and advice I received and direction. Regrettably, the opportunities identified to from elders and others and through learning our history. break down the barriers preventing progress are slow to I believe that, in part, our ability to successfully steer materialize. the BC treaty process through the next stage of its development requires some reflection on where we’ve During the past year, the provincial government and been and what we’ve learned. First Nations, both in and outside the treaty process have engaged in discussions on how more substantive and In 1994, the Treaty Commission issued its first annual tangible progress can be made on the ‘new relationship’. report which included this statement, “The Commission- Among the options explored was a recognition and ers recognize that the treaty making process holds great reconciliation act. -

Thursday, October 22, 2015
REGIONAL DISTRICT •OF BULKLEY-NECHAKO RURAL DIRECTORS COMMITTEE SUPPLEMENTARY AGENDA Thursday, October 22, 2015 PAGE NO. REPORT ACTION 2-31 Gail Chapman, Chief Administrative Officer Receive - Recreational SiteslAreas in the ROBN NEW BUSINESS ADJOURNMENT ( REGIONAL DISTRICT OF BULKLEY-NECHAKO Rural Directors Committee SUPPLEMENTARY MEMORANDUM TO: Chairperson Benedict and Committee Members FROM: Gail Chapman, Chief Administrative Officer DATE: October 21, 2015 SUBJECT: Recreational Sites/Areas in the RDBN In 2009, the RDBN completed a recreation inventory which includes the location and maintenance status of recreation sites which were previously maintained by the Ministry of Forests together with other recreation sites in the region. Also, mapping was created to identify the location of the sites. A copy of the 2009 memo will be attached as a Supplementary Agenda item for your reference. Mapping that identifies the location of sites has been developed and is also available should Rural Directors wish a copy. Currently, of the 28 BC Regional Districts, 22 of them manage regional parks in some form or another. There is no single widely accepted definition of a "regional park" across the province or country. Each Regional Park and Rec Trails Master Plan document in BC contains a different definition unique to its region. Commonalities include management guidelines, permitted or intended uses, and levels of protection. Staff contacted all Regional Districts in the Province in 2013 to inquire of Regional Districts if they have a Parks and -
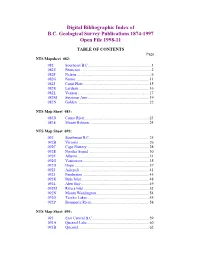
Digital Bibliographic Index of B.C. Geological Survey Publications 1874-1997 Open File 1998-11
Digital Bibliographic Index of B.C. Geological Survey Publications 1874-1997 Open File 1998-11 TABLE OF CONTENTS Page NTS Mapsheet 082: 082 Southeast B.C..............................................................1 082E Penticton.....................................................................2 082F Nelson.........................................................................6 082G Fernie........................................................................11 082J Canal Flats.................................................................15 082K Lardeau.....................................................................16 082L Vernon.......................................................................17 082M Seymour Arm............................................................19 082N Golden.......................................................................22 NTS Map Sheet 083: 083D Canoe River...............................................................23 083E Mount Robson...........................................................24 NTS Map Sheet 092: 092 Southwest B.C...........................................................25 092B Victoria.....................................................................26 092C Cape Flattery.............................................................28 092E Nootka Sound...........................................................30 092F Alberni.......................................................................31 092G Vancouver.................................................................35 -

85.445 NA-D042 Alabama Weiss Lake
State Lake Name County Contours LAT LON Region Alabama Barbour County Lake Barbour HD 31.968 -85.445 NA-D042 Alabama Weiss Lake Cherokee STD 34.215 -85.646 NA-D042 Alabama TomBigbee River Clarke STD 31.836 -88.165 NA-D042 Alabama Clay County Lake Clay HD 33.430 -85.703 NA-D042 Alabama Martin Lake Elmore STD 32.807 -85.895 NA-D042 Alabama Pickwick Lake Lauderdale STD 34.963 -88.177 NA-D042 Alabama Wheeler Lake Lauderdale STD 34.752 -87.299 NA-D042 Alabama Wilson Lake Lauderdale STD 34.819 -87.494 NA-D042 Alabama Madison County Lake Madison HD 34.810 -86.392 NA-D042 Alabama Guntersville Lake Marshall STD 34.475 -86.197 NA-D042 Alabama Logan Martin Lake Talladega STD 33.488 -86.261 NA-D042 Alabama Black Warrior River Tuscaloosa STD 32.974 -87.735 NA-D042 Alaska Alder Pond Anchorage STD 60.806 -148.937 NA-D029 Alaska APU University Lake Anchorage STD 61.186 -149.803 NA-D029 Alaska Beach Lake Anchorage STD 61.405 -149.559 NA-D029 Alaska Cheney Lake Anchorage STD 61.202 -149.760 NA-D029 Alaska Clunie Lake Anchorage STD 61.354 -149.611 NA-D029 Alaska Delong Lake Anchorage STD 61.162 -149.958 NA-D029 Alaska Dishno Pond Anchorage STD 61.235 -149.650 NA-D029 Alaska Eklutna Lake Anchorage STD 61.380 -149.044 NA-D029 Alaska Gwen Lake Anchorage STD 61.298 -149.678 NA-D029 Alaska Jewel Lake Anchorage STD 61.140 -149.963 NA-D029 Alaska Lake Otis Anchorage HD 61.191 -149.845 NA-D029 Alaska Lower Fire Lake Anchorage STD 61.353 -149.545 NA-D029 Alaska Mirror Lake Anchorage STD 61.426 -149.414 NA-D029 Alaska Otter Lake Anchorage STD 61.290 -149.736 NA-D029 -

The Grasslands of British Columbia
The Grasslands of British Columbia The Grasslands of British Columbia Brian Wikeem Sandra Wikeem April 2004 COVER PHOTO Brian Wikeem, Solterra Resources Inc. GRAPHICS, MAPS, FIGURES Donna Falat, formerly Grasslands Conservation Council of B.C., Kamloops, B.C. Ryan Holmes, Grasslands Conservation Council of B.C., Kamloops, B.C. Glenda Mathew, Left Bank Design, Kamloops, B.C. PHOTOS Personal Photos: A. Batke, Andy Bezener, Don Blumenauer, Bruno Delesalle, Craig Delong, Bob Drinkwater, Wayne Erickson, Marylin Fuchs, Perry Grilz, Jared Hobbs, Ryan Holmes, Kristi Iverson, C. Junck, Bob Lincoln, Bob Needham, Paul Sandborn, Jim White, Brian Wikeem. Institutional Photos: Agriculture Agri-Food Canada, BC Archives, BC Ministry of Forests, BC Ministry of Water, Land and Air Protection, and BC Parks. All photographs are the property of the original contributor and can not be reproduced without prior written permission of the owner. All photographs by J. Hobbs are © Jared Hobbs. © Grasslands Conservation Council of British Columbia 954A Laval Crescent Kamloops, B.C. V2C 5P5 http://www.bcgrasslands.org/ All rights reserved. No part of this document or publication may be reproduced in any form without prior written permission of the Grasslands Conservation Council of British Columbia. ii Dedication This book is dedicated to the Dr. Vernon pathfinders of our ecological Brink knowledge and understanding of Dr. Alastair grassland ecosystems in British McLean Columbia. Their vision looked Dr. Edward beyond the dust, cheatgrass and Tisdale grasshoppers, and set the course to Dr. Albert van restoring the biodiversity and beauty Ryswyk of our grasslands to pristine times. Their research, extension and teaching provided the foundation for scientific management of our grasslands. -
^ = Partial Bathymetric Coverage * = Detailed Shoreline Only Page 1 of 19
^ = Partial Bathymetric Coverage * = Detailed Shoreline Only Inland Lakes British Columbia #3 Lake BC Alta Lake BC Baptiste Lake BC 103 Mile Lake BC Amanita Lake BC Barbara Lake BC 108 Mile Lake BC Ambrose Lake BC Bardolph Lake BC 130 Mile Lake BC Amor Lake BC Barnes Lake BC Abas Lake BC Anahim Lake BC Barsby Lake (Blind Lake) BC Abbott Lake BC Anderson Lake BC Barton Lake BC Abel Lake BC Andy Bailey Lake BC Basalt Lake BC Aberdeen Lake BC Angler Lake BC Battleship Lake BC Abrams Lake BC Angly Lake BC Baynes Lake BC Abruzzi Lake BC Angora Lake BC Beale Lake BC Abuntlet Lake BC Ant Lake BC Bear Creek Reservoir BC Academus Lake BC Antler Lake BC Bear Lake BC Acorn Lake BC Antoine Lake BC Bearhole Lake BC Aeroplane Lake BC Anutz Lake BC Bearpaw Lake BC Ahdatay Lake BC Anzac Lake BC Beartrack Lake BC Aid Lake BC Anzus Lake BC Beartrap Lake BC Aiken Lake BC Arctic Lake BC Beatrice Lake BC Aird Lake BC Armstrong Lake BC Beaux Yeux Lake BC Airline Lake BC Ash Lake BC Beaver Lake BC Alah Lake BC Atan Lake BC Beaverlodge Lake BC Albert Head Lagoon BC Atlin Lake BC/YT Beavertail Lake BC Albert Lake BC Atluck Lake BC Beck Lake BC Alces Lake BC Augier Lake BC Becker Lake BC Alex Graham Lake BC Azouzetta Lake BC Bedingfield Lake BC Alexis Lake BC Azuklotz Lake BC Bednesti Lake BC Aleza Lake BC Azure Lake BC Begbie Lake BC Alice Lake BC Babcock Lake BC Belcourt Lake BC Allan Lake BC Babette Lake BC Bells Lake BC Allendale Lake BC Badger Lake BC Ben Lake BC Alleyne Lake BC Baile Lake BC Bennett Lake BC/YT Allison Lake BC Balfour Lake BC Benny Lake BC -

Xerox University Microfilms
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon die quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Pago(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again - beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

British Columbia's Grassland Regions
British Columbia’s Grassland Regions British Columbia’s Grassland Regions The Grasslands Conservation Council of British Columbia’s mission is to: • Foster understanding and appreciation for the ecological, social, economic, and cultural importance of BC grasslands. • Promote stewardship and sustainable management practices to ensure the long-term health of BC grasslands. • Promote the conservation of representative grassland ecosystems, species at risk, and their habitats. The GCC acknowledges the contributions of the original authors, artists, and photographers of the material in this e-book: staff members, contractors, and volunteers. Commonsense solutions for BC grasslands. bcgrasslands.org Grasslands Conservation Council of British Columbia. (2017). British Columbia’s Grassland Regions. Kamloops, BC: Author. © Grasslands Conservation Council of British Columbia 2 British Columbia’s Grassland Regions Table of Contents GRASSLANDS MAPPING .......................................................................................................................................... 4 Grassland Facts | Ecosystem Classification Systems MAJOR GRASSLAND ECOSYSTEMS ................................................................................................................. 6 Each ecosystem below includes: Grassland Landscapes | Unique Features | Plant Communities | Key Plant Species | Wildlife | Species at Risk East Kootenay Trench ................................................................................................................................................. -

31761 05013 5912 Contributions of the Royal
RO YAL M LIB 3 1 76 1 050 1 3 59 1 2 C O NT R IB U T IO NS OF T H E R OY AL O NT AR IO M U S EU M O F Z O O LO G Y o : S M E F R ES H - T ER I S H ES F B R I T I S H N . 9 O WA F O B Y DYM O D ‘ I R . CO LU M B A, J. N . ‘ Reprinted from Report of the Commissioner of Fisheries of B ritis h Columbid or the ear 1 f y 935 . R 1 9 3 E 5 . L 60 R E P O R T O F T H C O M M I S S I O N E R O F F I S H E I E S , - H O LU MB A S O M E FRES H WATER FI S HES O F B RITI S C I . D YM O D IR CTOR OYAL S M OF Z OOLOG Y T ORO TO O N T . B Y J . R . ND , E , R M U EU . , N , The f o llo wing notes are b a s e d la r g e ly o n specimens and in fo r ma t io n obtained in B ritish C f 2 6 9 2 o l umb ia during the summers o 1 9 and 1 8 , when the writer was engaged in a study of o f the trout and other game fishes of the Province . -

Schedule D: Flood Construction Level and Floodplain Setback Table
REGIONAL DISTRICT OF BULKLEY-NECHAKO FLOODPLAIN MANAGEMENT BYLAW NO. 1878, 2020 Schedule D: Flood Construction Level and Floodplain Setback Table • G.S.C means Geodetic Survey of Canada Datum. • Flood Construction Levels identified in metres are measured from the Natural Boundary of the waterbody at its location closest to the area of construction. • Flood Construction Levels are only applicable to Lands within the distance specified from the waterbody in the below table. Waterbody Above/below Flood Floodplain Distance Construction Setback Level Any other lake, marsh or pond 1.5 metres 7.5 m 100 m Any Watercourse 1.5 metres 15.0 m 100 m Any dike right of way, or structure for 7.5 m flood protection or seepage control Babine Lake 713.63 G.S.C 7.5 m 100 m Babine River 3.0 m 30.0 m 200 m Buck Creek 3.0 m 30.0 m 200 m Bulkley River Schedule A Bulkley River (where not covered by 3.0 m 30.0 m 200 m Schedule A) Cheslatta River 3.0 m 30.0 m 200 m Cheslatta/Murray Lakes 3.0 m 7.5 m 200 m Chilako River 3.0 m 30.0 m 200 m Chuchi Lake 3.0 m 7.5 m 200 m Cluculz Creek below Cluculz 3.0 m 30.0 m 200 m Lake Cluculz Lake 765.70 G.S.C 7.5 m 100 m Cunningham Lake 3.0 m 7.5 m 200 m Driftwood River 3.0 m 30.0 m 200 m Endako River 3.0 m 30.0 m 200 m Eutsuk Lake 3.0 m 7.5 m 200 m Fleming Creek below 3.0 m 30.0 m 200 m Tidesley Creek Francois Lake 3.96 on Water 7.5 m 100 m Survey of Canada gauge #08JB011 1 REGIONAL DISTRICT OF BULKLEY-NECHAKO FLOODPLAIN MANAGEMENT BYLAW NO.