SAM Status and Trends Mussel Monitoring Draft Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beaufort Sea Monitoring Program
Outer Continental Shelf Environmental Assessment Program Beaufort Sea Monitoring Program: Proceedings of a Workshop and Sampling Design Recommendations Beaufort Sea Monitoring Program: Proceedings of a Workshop (September 1983) and Sampling Design Recommendations ; Prepared for the Outer Continental Shelf Environmental Assessment Program Juneau, Alaska by J. P. Houghton Dames & Moore 155 N.E. lOOth Street Seattle, WA 98125 with D. A Segar J. E. Zeh SEAM Ocean Inc. Department of Statistics Po. Box 1627 University of Washington Wheaton, MD 20902 Seattle, WA 98195 April 1984 UNITED STATES UNITED STATES DEPARTMENT OF COMMERCE DEPARTMENT OF THE INTERIOR Malcolm Baldridge, Secretary William P Clark, Secretary NATIONAL OCEANIC AND MINERALS MANAGEMENT SERVICE ATMOSPHERIC ADMINISTRATION William D. Bettenberg, Director John V. Byrne, Administrator r. NOTICES i? I This report has been reviewed by the US. Department of Commerce, National Oceanic and Atmospheric Administration's Outer Continental Shelf Environmental Assessment Program office, and approved for publication. The interpretation of data and opinions expressed in this document are those of the authors and workshop participants. Approval does not necessarily signify that the contents reflect the views and policies of the Department of Commerce or those of the Department of the Interior. The National Oceanic and Atmospheric Administration (NOAA) does not approve, recommend, or endorse any proprietary product or proprietary material mentioned in this publica tion. No reference shall be made to NOAA or to this publication in any advertising or sales promotion which would indicate or imply that NOAA approves, recommends, or endorses any proprietary'product or proprietary material mentioned herein, or which has as its purpose an intent to cause directly or indirectly the advertised product to be used or purchas'ed because of this publication. -

Lake Michigan Pilot Study National Monitoring Network for U.S. Coastal
Lake Michigan Pilot Study of the National Monitoring Network for U.S. Coastal Waters and Their Tributaries A project of the Lake Michigan Monitoring Coordination Council and many Lake Michigan and Great Lakes partners for the National Water Quality Monitoring Network February, 2008 Table of Contents Introduction .................................................................................................................................3 I. Overview of the Study Area ................................................................................................4 Size and Characteristics of the Lake Michigan Watershed .......................................................4 Major Tributaries .......................................................................................................................5 Major Land and Resource Uses................................................................................................5 II. Major Management Issues ..................................................................................................6 Fish Consumption Advisories....................................................................................................7 Toxic Hot Spots – Great Lakes Areas of Concern ....................................................................8 Beach Closures .........................................................................................................................9 Drinking Water-Borne Illnesses...............................................................................................12 -

Tolmie State Park Washington State Parks • Park Hours – 7730 61St Ave NE Olympia, WA 98506 April 16 to Sept
Things to remember Tolmie State Park Washington State Parks • Park hours – 7730 61st Ave NE Olympia, WA 98506 April 16 to Sept. 15, (360) 456-6464 8 a.m. to dusk. • Winter schedule – Sept. 16 to State Parks information: (360) 902-8844 April 15, 8 a.m. to dusk, Wednesday through Sunday. Although most parks Reservations: Online at are open year round, some parks or portions of www.parks.state.wa.us or call TolmieState Park parks are closed during the winter. For a winter (888) CAMPOUT or (888) 226-7688 schedule and information about seasonal Other state parks located in closures, visit www.parks.state.wa.us or call the the general area: information center at (360) 902-8844. Eagle Island, Joemma Beach, Millersylvania and Penrose Point • Moorage fees are charged year round for mooring at docks, floats and buoys from 1 p.m. to 8 a.m. • Wildlife, plants and all park buildings, signs, tables and other structures are protected; removal Connect with us on social media or damage of any kind is prohibited. Hunting, www.twitter.com/WAStatePks feeding of wildlife and gathering firewood on state park property is prohibited. www.facebook.com/WashingtonStateParks • Pets must be on leash and under physical control www.youtube.com/WashingtonStateParks at all times. This includes trail areas and campsites. Share your stories and photos: Adventure Awaits.com Pet owners must clean up after pets on all state park lands. S Sample If you would like to support Washington State S Sample Parks even more, please consider making a 2018 donation when renewing your license plate tabs. -

Marine Ecology Progress Series 351:163
Vol. 351: 163–175, 2007 MARINE ECOLOGY PROGRESS SERIES Published December 6 doi: 10.3354/meps07033 Mar Ecol Prog Ser Structural and functional effects of Mytilus edulis on diversity of associated species and ecosystem functioning Pia Norling*, Nils Kautsky Department of Systems Ecology, Stockholm University, 106 91 Stockholm, Sweden ABSTRACT: Habitat-modifying species such as Mytilus edulis strongly impact both community struc- ture and ecosystem functioning through positive or negative interactions with other species and by changing physical and biological conditions. A study of natural patches of mussels showed that C and N content of sediment was higher in mussel patches compared to the surrounding sand community. Species richness and biomass of associated macrofauna and -flora was enhanced even by the pres- ence of single mussels and increased rapidly with patch size up to about 150 cm2, while no change in sediment meiofauna was found. In order to separate the effects of structure and function of M. edulis, a manipulative field experiment was performed with constructed patches of cleaned live mussels or intact empty shells. After 3 mo colonisation, the number of associated species in both treatments approached those in natural mussel patches, indicating that species richness was mainly due to phys- ical structure. Abundance and biomass of associated flora and fauna were higher if live mussels were present because mussel biodeposition and nutrient regeneration supplied limiting resources and increased carrying capacity. Species composition was also affected. Complexity and biodiversity increased with time, especially if Fucus vesiculosus plants established themselves. Measurements of community metabolism showed that the associated community found in mussel patches depends on mussel biodeposition for 24 to 31% of its energy demand. -

Genus Mytilus (Moflusca: Bivalvia)
Heredity74 (1995)369—375 Received26 May 1994 Chromosomal markers in three species of the genus Mytilus (Moflusca: Bivalvia) A. MARTINEZ-LAGE, A. GONZALEZ-TIZON & J. MENDEZ Departamento de Bio/ogia Ce/u/ar y Molecular, Areade Genética,Universidad de La Coruña, 15071 La Coruña, Spain Theanalysis of C-banding, NOR and fluorochrome staining was carried out in three species of European mussel, Mytilus edulis, M. galloprovincialis and M. trossulus. The results obtained allow us to detect changes in the constitutive heterochromatin within the genus Mytilus. The existence of chromosomal markers permit us to identify and distinguish, at the cytogenetical level, these three types of mussel. Keywords:C-bands,chromosome markers, CMA3 bands, heterochromatin, Mytilus, NORs. along various coastal regions of the British Isles. Subse- Introduction quently, in France, Britain and Ireland, M. galloprovin- Thegenus Mytilus is the subject of an important cialis has been found to be intermixed with M. edulis, controversy about the systematic status of the different producing hybrid forms (Skibinski & Beardmore, forms within it. In this genus, taxonomic studies were 1979). M. trossulus is distributed in the Baltic Sea. In initially developed in order to establish a systematic the Danish straits, M. trossulus is intermixed with M. relationship between M edulis and M. gaioprovincialis edulis (Váinölä & Hvilsom, 1991). and, later, among other forms of mussels. At first, the On the other hand, studies using Mytilus cyto- studies were focused on morphological criteria and genetics have shown that the diploid number is 28 morphometric parameters (internal and external shell chromosomes (Ahmed & Sparks, 1970; Ieyama & characteristics, the anterior adductor muscle features, Inaba, 1974; Thiriot-Quiévreux & Ayraud, 1982; etc.). -

First Record of the Mediterranean Mussel Mytilus Galloprovincialis (Bivalvia, Mytilidae) in Brazil
ARTICLE First record of the Mediterranean mussel Mytilus galloprovincialis (Bivalvia, Mytilidae) in Brazil Carlos Eduardo Belz¹⁵; Luiz Ricardo L. Simone²; Nelson Silveira Júnior³; Rafael Antunes Baggio⁴; Marcos de Vasconcellos Gernet¹⁶ & Carlos João Birckolz¹⁷ ¹ Universidade Federal do Paraná (UFPR), Centro de Estudos do Mar (CEM), Laboratório de Ecologia Aplicada e Bioinvasões (LEBIO). Pontal do Paraná, PR, Brasil. ² Universidade de São Paulo (USP), Museu de Zoologia (MZUSP). São Paulo, SP, Brasil. ORCID: http://orcid.org/0000-0002-1397-9823. E-mail: [email protected] ³ Nixxen Comercio de Frutos do Mar LTDA. Florianópolis, SC, Brasil. ORCID: http://orcid.org/0000-0001-8037-5141. E-mail: [email protected] ⁴ Universidade Federal do Paraná (UFPR), Departamento de Zoologia (DZOO), Laboratório de Ecologia Molecular e Parasitologia Evolutiva (LEMPE). Curitiba, PR, Brasil. ORCID: http://orcid.org/0000-0001-8307-1426. E-mail: [email protected] ⁵ ORCID: http://orcid.org/0000-0002-2381-8185. E-mail: [email protected] (corresponding author) ⁶ ORCID: http://orcid.org/0000-0001-5116-5719. E-mail: [email protected] ⁷ ORCID: http://orcid.org/0000-0002-7896-1018. E-mail: [email protected] Abstract. The genus Mytilus comprises a large number of bivalve mollusk species distributed throughout the world and many of these species are considered invasive. In South America, many introductions of species of this genus have already taken place, including reports of hybridization between them. Now, the occurrence of the Mediterranean mussel Mytilus galloprovincialis is reported for the first time from the Brazilian coast. Several specimens of this mytilid were found in a shellfish growing areas in Florianópolis and Palhoça, Santa Catarina State, Brazil. -
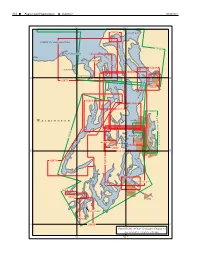
Chapter 13 -- Puget Sound, Washington
514 Puget Sound, Washington Volume 7 WK50/2011 123° 122°30' 18428 SKAGIT BAY STRAIT OF JUAN DE FUCA S A R A T O 18423 G A D A M DUNGENESS BAY I P 18464 R A A L S T S Y A G Port Townsend I E N L E T 18443 SEQUIM BAY 18473 DISCOVERY BAY 48° 48° 18471 D Everett N U O S 18444 N O I S S E S S O P 18458 18446 Y 18477 A 18447 B B L O A B K A Seattle W E D W A S H I N ELLIOTT BAY G 18445 T O L Bremerton Port Orchard N A N 18450 A 18452 C 47° 47° 30' 18449 30' D O O E A H S 18476 T P 18474 A S S A G E T E L N 18453 I E S C COMMENCEMENT BAY A A C R R I N L E Shelton T Tacoma 18457 Puyallup BUDD INLET Olympia 47° 18456 47° General Index of Chart Coverage in Chapter 13 (see catalog for complete coverage) 123° 122°30' WK50/2011 Chapter 13 Puget Sound, Washington 515 Puget Sound, Washington (1) This chapter describes Puget Sound and its nu- (6) Other services offered by the Marine Exchange in- merous inlets, bays, and passages, and the waters of clude a daily newsletter about future marine traffic in Hood Canal, Lake Union, and Lake Washington. Also the Puget Sound area, communication services, and a discussed are the ports of Seattle, Tacoma, Everett, and variety of coordinative and statistical information. -

Mussel Watchtand Chemical Contamination of the Coasts by Polycyclic Aromatic Hydrocarbons*
IAEA-SM-354/117 "MUSSEL WATCHTAND CHEMICAL CONTAMINATION OF THE COASTS BY POLYCYCLIC AROMATIC HYDROCARBONS* FARRINGTON, J. W. XA9951933 Woods Hole Oceanographic Institution 360 Woods Hole Road - MS#31 Woods Hole, Massachusetts 02543-1541 U. S. A. Abstract Polycyclic aromatic hydrocarbons (PAH) enter the coastal marine environment from three general categories of sources; pyrogenic, petrogenic (or petroleum), and natural diagenesis. PAH from different sources appear to have differential biological availability related to how the PAH are sorbed, trapped, or chemically bound to particulate matter, including soot. Experience to date with bivalve sentinel organism, or "Mussel Watch", monitoring programs indicates that these programs can provide a reasonable general assessment of the status and trends of biologically available PAH in coastal ecosystems. As fossil fuel use increases in developing countries, it is important that programs such as the International Mussel Watch Program provide assessments of the status and trends of PAH contamination of coastal ecosystems of these countries. 1. INTRODUCTION Modern societies have deliberately and inadvertently discharged or released chemicals of environmental concern to the coastal ocean for decades. The serious nature of several of the ensuing problems identified in the 1960s and early 1970s demonstrated the need for an organized and systematic approach to assessing the status and trends of contamination of coastal and estuarine ecosystems by selected chemicals of major concern. Surveys in the 1960s and early 1970s involving sampling and analysis of various components of coastal and estuarine ecosystems led to the conclusion that much could be learned about spatial and temporal trends by sampling and analyzing carefully chosen populations of bivalves. -

Occurrence of Contaminants of Emerging
Occurrence of contaminants of emerging concern in mussels (Mytilus spp.) along the California coast and the influence of land use, stormwater discharge, and treated wastewater effluent Nathan G. Dodder1, Keith A. Maruya1, P. Lee Ferguson2, Richard Grace3, Susan Klosterhaus4,*, Mark J. La Guardia5, Gunnar G. Lauenstein6 and Juan Ramirez7 results suggest that certain compounds; for example, ABSTRACT alkylphenols, lomefloxacin, and PBDE, are appropriate Contaminants of emerging concern were measured for inclusion in future coastal bivalve monitoring efforts in mussels collected along the California coast in 2009- based on maximum concentrations >50 ng/g dry weight 2010. The seven classes were alkylphenols, pharmaceu- and detection frequencies >50%. Other compounds, for ticals and personal care products, polybrominated diphe- example PFC and hexabromocyclododecane (HBCD), nyl ethers (PBDE), other flame retardants, current use may also be suggested for inclusion due to their >25% pesticides, perfluorinated compounds (PFC), and single detection frequency and potential for biomagnification. walled carbon nanotubes. At least one contaminant was detected at 67 of the 68 stations (98%), and 67 of the 167 analytes had at least one detect (40%). Alkylphenol, INTRODUCTION PBDE, and PFC concentrations increased with urbaniza- The National Oceanic and Atmospheric tion and proximity to stormwater discharge; pesticides Administration’s National Status and Trends (NOAA had higher concentrations at agricultural stations. These NS&T) Mussel Watch Program -

Shoreline Inventory and Characterization Report
Final Draft THURSTON COUNTY SHORELINE MASTER PROGRAM UPDATE Inventory and Characterization Report SMA Grant Agreements: G0800104 and G1300026 June 30, 2013 Prepared By: Thurston County Planning Department Building # 1, 2nd Floor 2000 Lakeridge Drive SW Olympia, WA 98502-6045 This page left intentionally blank. Table of Contents 1 INTRODUCTION ............................................................................................................................................ 1 REPORT PURPOSE .......................................................................................................................................................... 1 SHORELINE MASTER PROGRAM UPDATES FOR CITIES WITHIN THURSTON COUNTY ...................................................................... 2 REGULATORY OVERVIEW ................................................................................................................................................. 2 SHORELINE JURISDICTION AND DEFINITIONS ........................................................................................................................ 3 REPORT ORGANIZATION .................................................................................................................................................. 5 2 METHODS ..................................................................................................................................................... 7 DETERMINING SHORELINE JURISDICTION LIMITS .................................................................................................................. -

An Early Warning Program for Harmful Algal Blooms in Puget Sound
An Early Warning Program for Harmful Algal Blooms in Puget Sound Harmful Algae Puget Sound is one of the most naturally beautiful and ecologically diverse locations in the Pacific Northwest. However, these waters are home to several species of algae that can produce deadly toxins when water conditions encourage their populations to explode. During these harmful algal blooms (HABs), the algae and their toxins accumulate in shellfish, and are also transferred up the food chain to marine and terrestrial animals. High concentrations of some algal species can sicken or kill marine birds, mammals or humans and can cause fish to die in large numbers. The SoundToxins program We formed SoundToxins—the Partnership for Enhanced Monitoring and Emergency Response to HABs in Puget Sound—to combat the apparent increase in these HAB occurrences over the past decade. SoundToxins is a diverse partnership of shellfish farmers, fish farmers, environmental learning centers, volunteers, local health jurisdictions, colleges, and Native American tribes that was conceived and initiated by Northwest Fisheries Science Center (NWFSC), and is now co- directed by Washington Sea Grant (WSG). SoundToxins has grown from four partners in 2006 to over 28 partners in 2017, some of whom monitor multiple sites in Puget Sound. SoundToxins has three main objectives: • To document unusual bloom events and new species entering Puget Sound. • To determine the environmental conditions that promote the onset and flourishing of HABs. • To determine the combination of monitoring parameters that provide the earliest warnings of these events. Monitoring Puget Sound Pseudo-nitzschia SoundToxins samples phytoplankton, These algae are pennate diatoms that can produce a or single-celled algae, throughout toxin called domoic acid. -

Anderson and Ketron Islands Community Plan
Appendix B: Anderson - Ketron Islands Community Plan The Anderson - Ketron Islands Community Plan’s narrative text and policies are in addition to the Countywide Comprehensive Plan narrative text and policies and are only applicable within the Anderson-Ketron Islands Community Plan Boundary. • “Current” or “Existing” conditions are in reference to conditions at time of adoption (Adopted Ord. 2009-9s, Effective 6/1/2009). • “Proposed” or “Desired” conditions are those which required Council action and may have also been amended over time through a Comprehensive Plan Amendment (amendments are reflected in this document). CONTENTS Chapter 1: Introduction .................................................................................. B-6 Overview of the Plan Area ....................................................................................................... B-6 The Environment .................................................................................................................. B-7 History of Anderson Island....................................................................................................... B-7 Early History ......................................................................................................................... B-7 Early 20th Century ............................................................................................................... B-7 Industry, Commerce, and Services ...................................................................................... B-8 History of