Screening of Presence of the Chosen Anthocyanin Colorants in the Limniris Group Irises
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Complete Chloroplast Genomes Shed Light on Phylogenetic
www.nature.com/scientificreports OPEN Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Ju Namgung1,4, Hoang Dang Khoa Do1,2,4, Changkyun Kim1, Hyeok Jae Choi3 & Joo‑Hwan Kim1* Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, fve of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein‑coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA , psbA upstream, rpl32- trnL-UAG , ycf1, rpl22, matK, and ndhF, were identifed in the studied Allium species. Additionally, we present the frst phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, fve species of Amaryllidoideae, one species of Agapanthoideae, and fve species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae‑Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae‑ Gilliesieae‑Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by diferentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. -
Scanned Document
~ l ....... , .,. ... , •• 1 • • .. ,~ . · · . , ' .~ . .. , ...,.,, . ' . __.... ~ •"' --,~ ·- ., ......... J"'· ·····.-, ... .,,,.."" ............ ,... ....... .... ... ,,··~·· ....... v • ..., . .......... ,.. •• • ..... .. .. ... -· . ..... ..... ..... ·- ·- .......... .....JkJ(o..... .. I I ..... D · . ··.·: \I••• . r .• ! .. THE SPECIES IRIS STUDY GROUP OF THE AMERICAN IRIS SOCIETY \' -... -S:IGNA SPECIES IRIS GROUP OF NORTH AMERICA APRIL , 1986 NO. 36 OFFICERS CHAIRMAN: Elaine Hulbert Route 3, Box 57 Floyd VA 24091 VICE--CHAI.RMAN: Lee Welsr, 7979 W. D Ave. ~<alamazoo MI 4900/i SECRETARY: Florence Stout 150 N. Main St. Lombard, IL 6014~ TREASURER: Gene Opton 12 Stratford Rd. Berkelew CA 9470~ SEED EXCHANGE: Merry&· Dave Haveman PO Box 2054 Burling~rne CA 94011 -RO:E,IN DIRECTOR: Dot HuJsak 3227 So. Fulton Ave. Tulsc1, OK 74135 SLIDE DIRECTO~: Colin Rigby 2087 Curtis Dr . Penngrove CA 9495~ PUBLICATIONS SALES: Alan McMu~tr1e 22 Calderon Crescent Willowdale, Ontario, Canada M2R 2E5 SIGNA EDITOR : .Joan Cooper 212 W. Count~ Rd. C Roseville MN 55113 SIGNA PUBLISl-!ER:. Bruce Richardson 7 249 Twenty Road, RR 2 Hannon, Ontario, Canada L0R !Pe CONTENTS--APRIL, 1986--NO. 36 CHAIRMAN'S MESSAGE Elaine HL\l ber t 1261 PUBLICATI~NS AVAILABLE Al an McMwn tr ie 12c)1 SEED EXCHANGE REPORT David & Merry Haveman 1262 HONORARY LIFE MEMBERSHIPS El a ine? HLtlbert 1263 INDEX REPORTS Eric Tankesley-Clarke !263 SPECIES REGISTRATIONS--1985 Jean Witt 124-4' - SLIDE COLLECTION REPORT Col in Rigby 1264 TREASURER'S REPORT Gene (>pton 1264, NOMINATING COMMITTEE REPORT Sharon McAllister 1295 IRIS SOURCES UPDATE Alan McMurtrie 1266 QUESTIONS PLEASE '-Toan Cooper 1266 NEW TAXA OF l,P,IS L . FROM CHINA Zhao Yu·-· tang 1.26? ERRATA & ADDENDA ,Jim Rhodes 1269 IRIS BRAI\ICHil\iG IN TWO MOl~E SPECIES Jean Witt 1270 TRIS SPECIES FOR SHALLOW WATER Eberhard Schuster 1271 JAPANESE WILD IRISES Dr. -

Broadleigh Gardens 2014 Spring List
Broadleigh Gardens 2014 Spring list MAIL ORDER • 01823 286231 Bishops Hull • Taunton • Somerset TA4 1AE www.broadleighbulbs.co.uk Specialists in small bulbs Broadleigh Gardens Bishops Hull, Taunton, Somerset TA4 1AE Telephone: 01823 286231 Fax: 01823 323646 www.broadleighbulbs.co.uk “...they think warm days will never cease” aving been asked about my ‘retirement’ after Chelsea I thought you might like to see one of Hthe growing grandsons with the growing plants. The species peony collection is also growing and we hope Iris Double Lament Lilium Friso to have sufficient to offer more varieties soon. Things never stand still and one of the consequences of not doing Chelsea is that we no longer need some of the large show plants so this year we are able to offer the evergreen Dianella tasmanica (page 12) with its extraordinary blue berries. Some of our plants did not enjoy the wonderful summer as much as we did but the Schizostylis were an eye opener. They are stream side plants from southern Africa so we think of them as wanting dampish soils but forget that The youngest grandson - but Eucomis pole-evansii is winning! they experience seasonal rainfall and very hot summers. They literally blossomed and are still in full flower as I varieties are grown in an open field so we know they are write this in mid November. They are perfect to keep the hardy and we lift plants for sale. There are many more interest going into autumn I grow them in my dry ditch varieties on the website. with iris and hostas. -

Spring 2014 Cal-Sibe Siblings
Pacific Iris Almanac of the Society for Pacific Coast Native Iris www.pacificcoastiris.org Volume 42 No 2 Spring 2014 Cal-Sibe siblings 'Golden Waves', top, and 'Lyric Laughter', bottom, are sibling Cal-Sibes from the same cross, between a yellow-flowered seedling of I. forrestii and I. innominata. Jean Witt noted that the Siberian parent was a yellow 40 chromosome Siberian seedling, closer in form and color to I. forrestii than to I. wilsonii. The SIGNA Checklist states that the Siberian parent was I. wilsonii. Jean reviewed her notes, and said this is incorrect, it was a forrestii seedling. Year of registration: 1979 for ‗Golden Waves‘, 1988 for ‗Lyric Laughter‘, both by Jean Witt Photographs: Jean Witt Pacific Iris, Almanac of the Society for Pacific Coast Native Iris Volume XXXX1I Number 2 Spring 2014 SPCNI MEMBERSHIP The Society for Pacific Coast Native Irises (SPCNI) is a section of the American Iris Society (AIS). Membership in AIS is recommended but not required for membership in SPCNI. US Overseas Annual, paper $15.00 $18.00 Triennial, paper $40.00 $48.00 Annual, digital $7.00 $7.00 Triennial, digital $19.00 $19.00 Lengthier memberships are no longer available. Please send membership fees to the SPCNI Treasurer. Use Paypal to join SPCNI online at http://pacificcoastiris.org/JoinOnline.htm International currencies accepted IMPORTANT INFORMATION FROM THE SECRETARY/TREASURER ABOUT DUES NOTICES Members who get paper copies, please keep track of the expiration date of your member- ship, which is printed on your Almanac address label. We include a letter with your last issue, and may follow this with an email notice, if you have email. -

Vol. 49 Valencia, X-2011 FLORA MONTIBERICA
FLORA MONTIBERICA Publicación periódica especializada en trabajos sobre la flora del Sistema Ibérico Vol. 49 Valencia, X-2011 FLORA MONTIBERICA Publicación independiente sobre temas relacionados con la flora y la vegetación (plantas vasculares) de la Península Ibérica, especialmente de la Cordillera Ibérica y tierras vecinas. Fundada en diciembre de 1995, se publican tres volúmenes al año con una periodicidad cuatrimestral. Editor y Redactor general: Gonzalo Mateo Sanz. Jardín Botánico. Universidad de Valencia. C/ Quart, 80. E-46008 Valencia. Redactores adjuntos: Javier Fabado Alós. Redactor página web y editor adjunto: José Luis Benito Alonso. Edición en Internet: www.floramontiberica.org Flora Montiberica.org es la primera revista de botánica en español que ofrece de forma gratuita todos sus contenidos a través de la red. Consejo editorial: Antoni Aguilella Palasí (Universidad de Valencia) Juan A. Alejandre Sáenz (Herbarium Alejandre, Vitoria) Vicente J. Arán Redó (Consejo Superior de Investigaciones Científicas, Madrid) Manuel Benito Crespo Villalba (Universidad de Alicante) José María de Jaime Lorén (Universidad Cardenal Herrera-CEU, Moncada) Emilio Laguna Lumbreras ((Departamento de Medio Ambiente. Gobierno de la Comunidad Valenciana) Pedro Montserrat Recoder (Consejo Superior de Investigaciones Científicas, Jaca). Edita: Flora Montiberica. Valencia (España). ISSN: 1138-5952 – ISSN edición internet: 1988-799X. Depósito Legal: V-5097-1995. Portada: Ophioglossum azoricum C. Presl, procedente de Sotorribas (Cuenca). Véase pág. 36 de este número. Flora Montiberica 49: 3-5 (X-2011). ISSN 1988-799X NUEVA LOCALIDAD VALENCIANA DE PUCCINELLIA HISPANICA JULIÀ & J. M. MONTSERRAT (POACEAE) P. Pablo FERRER GALLEGO1 & Roberto ROSELLÓ GIMENO2 1Servicio de Biodiversidad, Centro para la Investigación y la Experimentación Forestal de la Generalitat Valenciana (CIEF). -

Alpine News, September 2008
Issue 23 • September 2008 AlpinenewsThe BIG Society for small plants! Newsletter of the Alpine Garden Society Free DVD with this Issue f the o en Enclosed with this issue is a DVD that contains the in ard et G l e y first 11 volumes of ‘The Alpine Gardener’ from 1930 l n et u i ci lp o to 1943. These are in the form of PDF files that are B S A fully searchable. Using Adobe Acrobat Reader you Volume 1-11 can search for an item within a volume or across all (1930-43) volumes on the disc - brief instructions are given in ‘The Alpine Gardener’ section of the AGS Website. www.alpinegardensociety.net C Alpine Garden Society 2008 AGS_CD.indd 1 23/7/08 16:06:01 IN THIS Online Flower show. ISSUE Please see page 5 for the details AGM Details of this years online flower show page 2 Seed Exchange page 12 Switch to New Paper Special Book This Newsletter is now printed on paper that is 50% recycled British Offers & New waste. 100% genuine printed waste is Titles used in the recycling process helping to page 14 reduce the pressure on UK landfill sites. The other 50% non-recycled wood Tours pulps used in this paper comes from Programme PEFC and FSC certified forests. page 22 AGS © 2008 Tel: 01386 554790 Noticeboard Annual General Meeting Saturday, 8 November 2008 Notice is hereby given that the Annual General Meeting of the Alpine Garden Society will be held at 11.00am on Saturday, 8 November, at the Stratford Manor Hotel, Warwick Road, Stratford-upon-Avon, Warwickshire, CV37 0PY. -

Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes
Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes Shichao Chen1., Dong-Kap Kim2., Mark W. Chase3, Joo-Hwan Kim4* 1 College of Life Science and Technology, Tongji University, Shanghai, China, 2 Division of Forest Resource Conservation, Korea National Arboretum, Pocheon, Gyeonggi- do, Korea, 3 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, United Kingdom, 4 Department of Life Science, Gachon University, Seongnam, Gyeonggi-do, Korea Abstract Phylogenetic analysis aims to produce a bifurcating tree, which disregards conflicting signals and displays only those that are present in a large proportion of the data. However, any character (or tree) conflict in a dataset allows the exploration of support for various evolutionary hypotheses. Although data-display network approaches exist, biologists cannot easily and routinely use them to compute rooted phylogenetic networks on real datasets containing hundreds of taxa. Here, we constructed an original neighbour-net for a large dataset of Asparagales to highlight the aspects of the resulting network that will be important for interpreting phylogeny. The analyses were largely conducted with new data collected for the same loci as in previous studies, but from different species accessions and greater sampling in many cases than in published analyses. The network tree summarised the majority data pattern in the characters of plastid sequences before tree building, which largely confirmed the currently recognised phylogenetic relationships. Most conflicting signals are at the base of each group along the Asparagales backbone, which helps us to establish the expectancy and advance our understanding of some difficult taxa relationships and their phylogeny. -

Korean Plants Paeonia
Korean plants Paeonia We had the opportunity for several hikes in National Parks while on Jeju Island where we were able to see many native Korean plants. Neillia (Stephanandra) incisa was one of the most common flowering shrubs in native areas Korea. It is cultivated in the US, but only as the cultivar ‘Crispa’. Rosa luciae produced large thickets of white flowers. Korean blackberry (Rubus coreanus) was in flower in the foothills on one of our hikes on Jeju Island. The fruit are made into a Korean fruit wine (bokbunia ju). Acer pseudosieboldianum While hiking on Jeju Island it was wonderful to realize that we were walking through native stands of hinoki cypress (Chamaecyparis obtusa). Daphniphyllum macropodum was an understory evergreen plant seen on our hikes on Jeju Island. It produces male and female flowers on separate plants. Female flowers Male flowers Viola mandshurica was common along the sides of the trail. Mukdenia rossii is a Korean plant just starting to gain popularity in the US as a shade-loving perennial. We were surprised to see so many Arisaema ringens plants growing in woodland areas. Korean jack-in-the-pulpit (Arisaema amurense serratum). Bijarim Forest on Jeju Island has the largest natural stand of nutmeg yew (Torreya nucifera). The oldest tree is over 800 years-old. There were several interesting plants along the coastline. Pittosporum tobira is native to Korea and a commonly cultivated shrub in southeastern and western USA. It is salt tolerant and common along the coastline. Beach silvertop (Glehnia littoralis) formed interesting rosettes of white color. We saw Korean pines (Pinus koraiensis) in the hills around the Hwagyesa Temple in Seoul and then again in the market where it is sold for its edible seeds. -
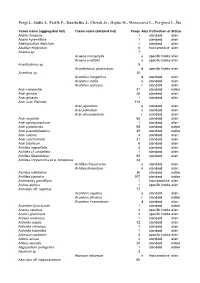
Pergl J., Sádlo J., Petřík P., Danihelka J., Chrtek Jr., Hejda M., Moravcová L., Perglová I., Štajerová K
Pergl J., Sádlo J., Petřík P., Danihelka J., Chrtek Jr., Hejda M., Moravcová L., Perglová I., Štajerová K. & Pyšek P. (2016): Dark side of the fence: ornamental plants as a source of wildgrowing flora in the Czech Republic. – Preslia 88: 163–184. Taxon name (aggregated list) Taxon name (detailed list) FrequencyAbundanceCultivation within demands aggregatedStatus taxa Abelia chinensis 1 standard alien Abelia ×grandiflora 1 standard alien Abeliophyllum distichum 1 standard alien Abutilon ×hybridum 6 frost sensitive alien Acaena sp. 7 Acaena microphylla e specific habitatalien Acaena ovalifolia e specific habitatalien Acantholimon sp. 9 Acantholimon glumaceum d specific habitatalien Acanthus sp. 10 Acanthus hungaricus d standard alien Acanthus mollis e standard alien Acanthus spinosus r standard alien Acer campestre 31 standard native Acer ginnala 28 standard alien Acer griseum 1 standard alien Acer sect. Palmata 113 Acer japonicum e standard alien Acer palmatum e standard alien Acer shirasawanum r standard alien Acer negundo 55 standard alien Acer pennsylvanicum 1 standard alien Acer platanoides 68 standard native Acer pseudoplatanus 39 standard native Acer rubrum 3 standard alien Acer saccharinum 21 standard alien Acer tataricum 6 standard alien Achillea ageratifolia 3 standard alien Achillea cf. umbellata 1 standard alien Achillea filipendulina 83 standard alien Achillea chrysocoma et A. tomentosa 23 Achillea chrysocoma e standard alien Achillea tomentosa e standard alien Achillea millefolium 36 standard native Achillea ptarmica 107 standard -

Nomenclatural Types of Iberian Irises (Iris and Related Genera, Iridaceae)
Flora Montiberica 53: 49-62 (18-XII-2012). ISSN: 1988-799X NOMENCLATURAL TYPES OF IBERIAN IRISES (IRIS AND RELATED GENERA, IRIDACEAE) Manuel B. CRESPO VILLALBA CIBIO, Instituto de la Biodiversidad. Universidad de Alicante. P.O. Box 99. E-03080 Alicante. E-mail: [email protected] ABSTRACT: Nomenclatural types are reported for seventeen taxa belonging to Iris and six related genera, which are accepted in the forthcoming treatment of Iridaceae for Flora iberica. Among them, 13 lectotypes and one neotype are designated for the first time, and three previous typifications are briefly commented. Keywords: Iris, Chamaeiris, Juno, Limniris, Xiphion, Hermodactylus, Gynandriris, nomenclature, typi- fication, Iberian Peninsula. RESUMEN: Tipos nomenclaturales de lirios ibéricos (Iris y géneros relaciona- dos, Iridaceaae). Se presentan los tipos nomenclaturales de 17 táxones pertenecientes a Iris y otros seis géneros relacionados, que se aceptan en el tratamiento de las Iridace- ae para Flora iberica. De ellos, se designan por primera vez 13 lectótipos y un neótipo, y se comentan brevemente tres tipificaciones previas. Palabras clave: Iris, Chamaei- ris, Juno, Limniris, Xiphion, Hermodactylus, Gynandriris, nomenclatura, tipificación, Península Ibérica. INTRODUCTION others), whereas others were accepted as separate genera (cf. PARLATORE, 1860; Iridaceae will be included in the KLATT, 1864, 1866; BAKER, 1877; forthcoming volume XX of Flora iberica. VALENTINE, 1980; RODIONENKO, As a part of the editorial task, data on no- 1961, 2005, 2007, 2009; MAVRODIEV, menclatural types will be reported for all 2010; among others). accepted taxa in the family. Some of the In any case, important morphological species occurring in the Iberian Peninsula differences exist among those seven ag- have already been typified, though many gregates, which allow recognition of uni- irises are still in need of typification. -

Secondary Metabolites of the Choosen Genus Iris Species
ACTA UNIVERSITATIS AGRICULTURAE ET SILVICULTURAE MENDELIANAE BRUNENSIS Volume LX 32 Number 8, 2012 SECONDARY METABOLITES OF THE CHOOSEN GENUS IRIS SPECIES P. Kaššák Received: September 13,2012 Abstract KAŠŠÁK, P.: Secondary metabolites of the choosen genus iris species. Acta univ. agric. et silvic. Mendel. Brun., 2012, LX, No. 8, pp. 269–280 Genus Iris contains more than 260 species which are mostly distributed across the North Hemisphere. Irises are mainly used as the ornamental plants, due to their colourful fl owers, or in the perfume industry, due to their violet like fragrance, but lot of iris species were also used in many part of the worlds as medicinal plants for healing of a wide spectre of diseases. Nowadays the botanical and biochemical research bring new knowledge about chemical compounds in roots, leaves and fl owers of the iris species, about their chemical content and possible medicinal usage. Due to this researches are Irises plants rich in content of the secondary metabolites. The most common secondary metabolites are fl avonoids and isofl avonoids. The second most common group of secondary metabolites are fl avones, quinones and xanthones. This review brings together results of the iris research in last few decades, putting together the information about the secondary metabolites research and chemical content of iris plants. Some clinical studies show positive results in usage of the chemical compounds obtained from various iris species in the treatment of cancer, or against the bacterial and viral infections. genus iris, secondary metabolites, fl avonoids, isofl avonoids, fl avones, medicinal plants, chemical compounds The genus Iris L.