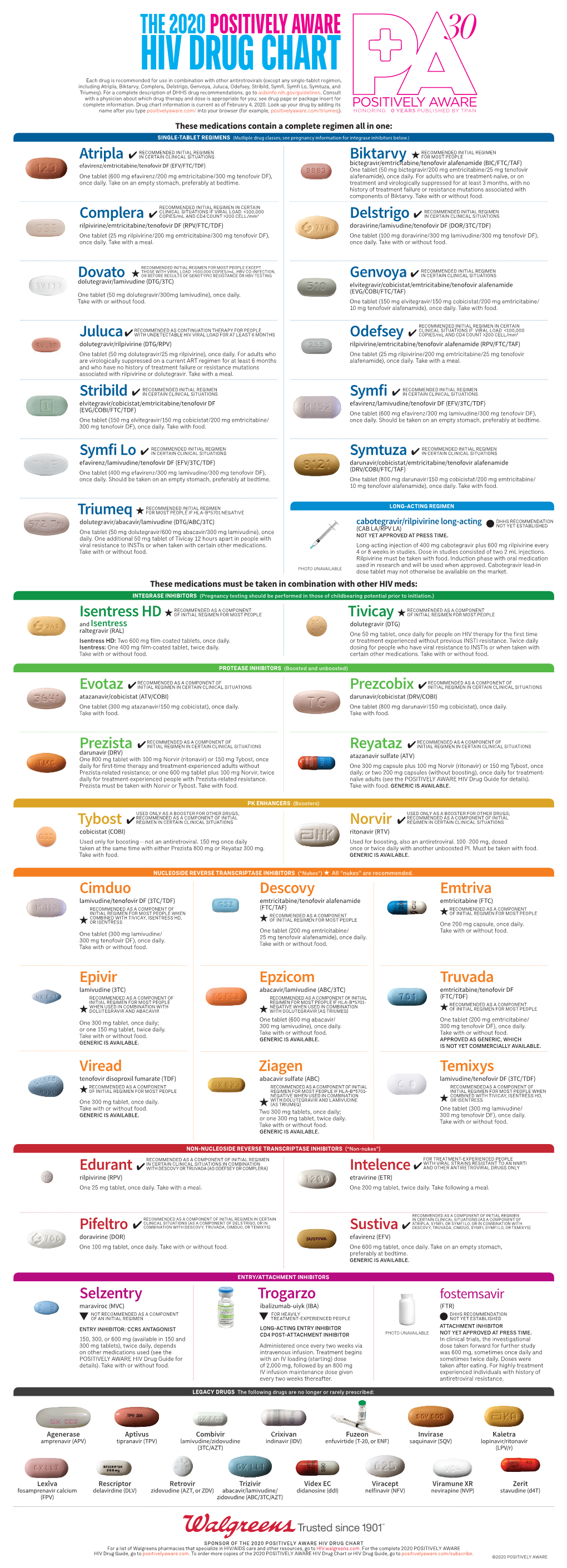
HIV Drug Chart 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Future of HIV Prevention and Treatment for Youth #Strive2optimize
THE AMERICAN ACADEMY OF HIV MEDICINE www.aahivm.org DECEMBER 2019 Patient Care, Practice Management & Professional Development Information for HIV Care Providers HIVSpecialist Integrating Transgender Healthcare for 24 The Future of Adolescents Not Your Parents’ HIV Prevention and Sex Talks 30 Treatment for Youth Adapting the Clinical Response 34 Fear of Addressing Substance Use and 36 Addiction DURABLE POWER AT PRESCRIBED REGIMEN FOR HIV-1 TREATMENT WEEK 144 Source: Ipsos Healthcare US HIV Therapy Monitor & Scope Study May-July 2019. BIKTARVY® (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg) combines the FTC/TAF* backbone with bictegravir, a novel and unboosted INSTI—for a powerful STR1,2 Learn more about the BIKTARVY 144 week data at BIKTARVY144.com Long-term e cacy in treatment-naïve adults at Week 1442-7 Results noninferior to comparators No treatment-emergent resistance associated with BIKTARVY through Week 1442-7 Study 1489: Virologic Response Study 1490: Virologic Response Week 48 Week 144 Week 48 Week 144 -0.6% (-4.8% to -2.6% (-8.5% to -3.5% (-7.9% to -1.9% (-7.8% to 1.0%; p=0.12)† 3.9%; p=0.52)† 100 3.6%; p=0.78)† 3.4%; p=0.39)† 100 HIV-1 RNA HIV-1 RNA <50 copies/mL <50 copies/mL % % % 92 93 % 93 80 % 80 89 % CASES 82% 84 82% 84 OF RESISTANCE WITH 60 60 BIKTARVY ve Adults, % Adults, ve ve Adults, % Adults, ve ï ï 0 40 40 In two large phase 3 clinical trials in treatment-naïve adults 20 20 • Among 634 treatment-naïve adults in Studies 1489 and 1490, 8 treatment-failure subjects were Treatment-Na Treatment-Na 0 0 tested and no amino acid substitutions emerged that were associated with BIKTARVY resistance Virologic failure Virologic failure HIV-1 RNA 1% 3% 1% 3% HIV-1 RNA 4% 1% 5% 3% ≥50 copies/mL ≥50 copies/mL BIKTARVY ABC/DTG/3TC BIKTARVY FTC/TAF+DTG urine glucose, and urine protein in all patients (n=314) (n=315) (n=320) (n=325) IMPORTANT SAFETY INFORMATION (cont’d) Contraindications as clinically appropriate. -

Bictegravir-Tenofovir Alafenamide-Emtricitabine (Biktarvy)
© National HIV Curriculum PDF created September 25, 2021, 12:10 pm Bictegravir-Tenofovir alafenamide-Emtricitabine (Biktarvy) Table of Contents Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy Summary Drug Summary Key Clinical Trials Key Drug Interactions Figures Drug Summary Bictegravir-tenofovir alafenamide-emtricitabine is a single-tablet regimen that is comprised of an integrase strand transfer inhibitor (bictegravir) combined with two nucleoside reverse transcriptase inhibitors (tenofovir alafenamide and emtricitabine). Bictegravir, which is not FDA-approved as an individual antiretroviral medication, has a high barrier to resistance, is well tolerated, and, extrapolating from the phase 3 data, has virologic efficacy comparable to dolutegravir. Bictegravir levels can be reduced with medications or oral supplement that contain polyvalent cations (e.g. Ca, Mg, Al, Fe). Bictegravir blocks tubular secretion of creatinine and typically raises serum creatinine by about 0.1 mg/dL, but without affecting renal glomerular function. Phase 3 trials in antiretroviral-naïve persons as well as switch trials in persons with virologic suppression have shown excellent efficacy with bictegravir-tenofovir alafenamide-emtricitabine. Available data suggest that bictegravir has a high genetic barrier to resistance. Key Clinical Trials In a phase 3 trial, 629 antiretroviral-naïve adults were randomized to receive bictegravir-tenofovir alafenamide-emtricitabine or dolutegravir-abacavir-lamivudine; after 48 weeks, there was no significant difference -

Guidelines for the Use of Antiretroviral Agents in Adults and Adolescent Living With
Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ AdultandAdolescentGL.pdf. Accessed [insert date] [insert page number, table number, etc. if applicable] It is emphasized that concepts relevant to HIV management evolve rapidly. The Panel has a mechanism to update recommendations on a regular basis, and the most recent information is available on the HIVinfo Web site (http://hivinfo.nih.gov). What’s New in the Guidelines? August 16, 2021 Hepatitis C Virus/HIV Coinfection • Table 18 of this section has been updated to include recommendations regarding concomitant use of fostemsavir or long acting cabotegravir plus rilpivirine with different hepatitis C treatment regimens. June 3, 2021 What to Start • Since the release of the last guidelines, updated data from the Botswana Tsepamo study have shown that the prevalence of neural tube defects (NTD) associated with dolutegravir (DTG) use during conception is much lower than previously reported. Based on these new data, the Panel now recommends that a DTG-based regimen can be prescribed for most people with HIV who are of childbearing potential. Before initiating a DTG-based regimen, clinicians should discuss the risks and benefits of using DTG with persons of childbearing potential, to allow them to make an informed decision. -

Management of Hiv Infection
MANAGEMENT OF HIV INFECTION Federal Bureau of Prisons Clinical Guidance September 2018 Clinical guidance is made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant this guidance for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Referenced Program Statement versions within this document are for informational purposes only. Please refer to the most current version of the referenced Program Statement. Consult the BOP Health Management Resources Web page to determine the date of the most recent update to this document: http://www.bop.gov/resources/health_care_mngmt.jsp Federal Bureau of Prisons Management of HIV Infection Clinical Guidance September 2018 WHAT’S NEW IN THIS DOCUMENT? This document updates the December 2017 version of the BOP Clinical Guidance on the Management of HIV Infection. Treatment information throughout this document was updated to be in line with the Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents issued by the Department of Health and Human Services (DHHS) in 2018. NOTEWORTHY CHANGES INCLUDE REVISIONS IN THE FOLLOWING AREAS: • INDICATIONS FOR HIV TESTING: An opt out strategy of voluntary testing for HIV infection is recommended for all inmates, regardless of sentencing status, including new intakes and those already established in the population who have not declined testing. • MENINGOCOCCAL VACCINE: Adults with HIV infection who have not been previously vaccinated should receive a 2-dose primary series of MenACWY (MCV4), available as Menactra® or Menveo®, at least 2 months apart, and then revaccinated every 5 years. -

BIKTARVY (Bictegravir, Emtricitabine, and Tenofovir
HIGHLIGHTS OF PRESCRIBING INFORMATION or below 15 mL per minute who have no antiretroviral treatment These highlights do not include all the information needed to use history. (2.3) BIKTARVY safely and effectively. See full prescribing information • Hepatic impairment: BIKTARVY is not recommended in patients for BIKTARVY. with severe hepatic impairment. (2.4) BIKTARVY® (bictegravir, emtricitabine, and tenofovir alafenamide) ----------------------DOSAGE FORMS AND STRENGTHS--------------------- tablets, for oral use Tablets: 50 mg of bictegravir (equivalent to 52.5 mg of bictegravir Initial U.S. Approval: 2018 sodium), 200 mg of emtricitabine, and 25 mg of tenofovir alafenamide (equivalent to 28 mg of tenofovir alafenamide fumarate). (3) WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B -------------------------------CONTRAINDICATIONS------------------------------- See full prescribing information for complete boxed warning. BIKTARVY is contraindicated to be co-administered with: • dofetilide. (4) Severe acute exacerbations of hepatitis B have been • rifampin. (4) reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing -----------------------WARNINGS AND PRECAUTIONS------------------------ emtricitabine (FTC) and/or tenofovir disoproxil fumarate • Immune reconstitution syndrome: May necessitate further evaluation (TDF), and may occur with discontinuation of BIKTARVY. and treatment. (5.3) Closely monitor hepatic function in these patients. If • New onset or worsening renal impairment: -

Bictegravir, Emtricitabine, and Tenofovir Alafenamide | Memorial
PATIENT & CAREGIVER EDUCATION Bictegravir, Emtricitabine, and Tenofovir Alafenamide This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Biktarvy Brand Names: Canada Biktarvy Warning Hepatitis B has gotten worse when this drug was stopped in some people with hepatitis B. Close follow-up for a few months is needed when therapy is stopped in people who have hepatitis B. Do not stop taking this drug without calling your doctor. What is this drug used for? It is used to treat HIV infection. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Kidney disease or liver disease. Bictegravir, Emtricitabine, and Tenofovir Alafenamide 1/6 If you are taking any of these drugs: Dofetilide, rifabutin, rifampin, rifapentine, or St. John’s wort. If you are taking any other drugs to treat HIV. If you are taking any drugs that can raise the chance of kidney problems. There are many drugs that can do this. Ask your doctor or pharmacist if you are not sure. If you are breast-feeding. Do not breast-feed while you take this drug. This is not a list of all drugs or health problems that interact with this drug. -

Bictegravir 50Mg/Emtricitabine 200Mg/Tenofovir Alafenamide 25Mg (Biktarvy®) with Other Antiretrovirals
FINAL VERSION – 2020-MAR -26 Title: Bictegravir 50mg/emtricitabine 200mg/tenofovir alafenamide 25mg (Biktarvy®) with other antiretrovirals Issue Statement Bictegravir 50mg/emtricitabine 200mg/tenofovir alafenamide 25mg (Biktarvy®) is approved by Health Canada for use as a complete regimen for the treatment of HIV-1 infection in adults with no known resistance to the components of the product [Biktarvy® Product Monograph {PM}]. Coadministration of Biktarvy® in combination with other antiretroviral agents is therefore off-label. However, we recogniZe that such use may be considered under specific circumstances, e.g. in patients with multidrug-resistant HIV-1 who require a multi-class antiretroviral regimen to achieve or maintain virologic suppression. The BC-CfE has received a number of requests for Biktarvy® in combination with other antiretrovirals; therefore, we require a consistent, evidence-based approach to handle such prescriptions. Background Biktarvy® is a three-drug fixed-dose combination containing bictegravir 50mg, emtricitabine 200mg, and tenofovir alafenamide 25mg [Biktarvy® PM]. Because Biktarvy® was developed as a complete single-tablet regimen, little information is available regarding its use with concomitant antiretrovirals. The potential safety and efficacy of such regimens could be impacted by drug-drug interactions between the component drugs of Biktarvy® and the concomitant antiretrovirals. The pharmacokinetic properties of tenofovir alafenamide (TAF) and bictegravir are reviewed below. Emtricitabine (FTC) is not expected to contribute significantly to drug-drug interactions with protease inhibitors (PIs) or nonnucleoside reverse transcriptase inhibitors (NNRTIs) [University Health Network {UHN}/Toronto General Hospital {TGH}; Liverpool HIV Drug Interactions website {Liverpool}]. Two fixed-dose combinations of FTC and TAF are available and approved by Health Canada [Descovy® PM]: o FTC/TAF 200/25 mg is recommended when used in combination with NNRTIs, unboosted integrase inhibitors, or unboosted ataZanavir. -

Proprietary Product Name: Biktarvy Sponsor: Gilead Sciences
Australian Public Assessment Report for Bictegravir / Emtricitabine / Tenofovir alafenamide Proprietary Product Name: Biktarvy Sponsor: Gilead Sciences August 2019 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website < https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

Drug Consumption at Wholesale Prices in 2017 - 2020
Page 1 Drug consumption at wholesale prices in 2017 - 2020 2020 2019 2018 2017 Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. ATC code Subgroup or chemical substance price/1000 € % price/1000 € % price/1000 € % price/1000 € % A ALIMENTARY TRACT AND METABOLISM 321 590 7 309 580 7 300 278 7 295 060 8 A01 STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01A STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01AA Caries prophylactic agents 663 8 611 11 619 12 1 042 11 A01AA01 sodium fluoride 610 8 557 12 498 15 787 14 A01AA03 olaflur 53 1 54 1 50 1 48 1 A01AA51 sodium fluoride, combinations - - - - 71 1 206 1 A01AB Antiinfectives for local oral treatment 1 266 10 1 101 6 1 052 6 944 6 A01AB03 chlorhexidine 930 6 885 7 825 7 706 7 A01AB11 various 335 21 216 0 227 0 238 0 A01AB22 doxycycline - - 0 100 0 100 - - A01AC Corticosteroids for local oral treatment 113 1 153 1 135 1 143 1 A01AC01 triamcinolone 113 1 153 1 135 1 143 1 A01AD Other agents for local oral treatment 49 0 72 0 104 0 - - A01AD02 benzydamine 49 0 72 0 104 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 30 885 4 32 677 4 35 102 5 37 644 7 A02A ANTACIDS 3 681 1 3 565 1 3 357 1 3 385 1 A02AA Magnesium compounds 141 22 151 22 172 22 155 19 A02AA04 magnesium hydroxide 141 22 151 22 172 22 155 19 A02AD Combinations and complexes of aluminium, 3 539 0 3 414 0 3 185 0 3 231 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 3 539 0 3 414 0 3 185 0 3 231 0 A02B DRUGS FOR PEPTIC ULCER AND 27 205 5 29 112 4 31 746 5 34 258 8 -

HIV-1 Integrase Tetramers Are the Antiviral Target of Pyridine-Based
RESEARCH ARTICLE HIV-1 integrase tetramers are the antiviral target of pyridine-based allosteric integrase inhibitors Pratibha C Koneru1, Ashwanth C Francis2, Nanjie Deng3, Stephanie V Rebensburg1, Ashley C Hoyte1, Jared Lindenberger1, Daniel Adu-Ampratwum4, Ross C Larue4, Michael F Wempe5, Alan N Engelman6,7, Dmitry Lyumkis8, James R Fuchs4, Ronald M Levy9, Gregory B Melikyan2, Mamuka Kvaratskhelia1* 1Division of Infectious Diseases, School of Medicine, University of Colorado, Aurora, United States; 2Division of Infectious Diseases, Department of Pediatrics, Emory University, Atlanta, United States; 3Department of Chemistry and Physical Sciences, Pace University, New York, United States; 4College of Pharmacy, The Ohio State University, Columbus, United States; 5Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Denver, Aurora, United States; 6Department of Cancer Immunology and Virology, Dana-Farber Cancer Institute, Boston, United States; 7Department of Medicine, Harvard Medical School, Boston, United States; 8Laboratory of Genetics, The Salk Institute for Biological Studies, La Jolla, United States; 9Department of Chemistry, Temple University, Philadelphia, United States Abstract Allosteric HIV-1 integrase (IN) inhibitors (ALLINIs) are a promising new class of antiretroviral agents that disrupt proper viral maturation by inducing hyper-multimerization of IN. Here we show that lead pyridine-based ALLINI KF116 exhibits striking selectivity for IN tetramers *For correspondence: versus lower order protein oligomers. IN structural features that are essential for its functional mamuka.kvaratskhelia@ucdenver. tetramerization and HIV-1 replication are also critically important for KF116 mediated higher-order edu IN multimerization. Live cell imaging of single viral particles revealed that KF116 treatment during Competing interest: See virion production compromises the tight association of IN with capsid cores during subsequent page 22 infection of target cells. -

The Emerging Role of HIV-1 Integrase in Virion Morphogenesis
viruses Review Going beyond Integration: The Emerging Role of HIV-1 Integrase in Virion Morphogenesis Jennifer L. Elliott and Sebla B. Kutluay * Department of Molecular Microbiology, Washington University School of Medicine, Saint Louis, MO 63110, USA; [email protected] * Correspondence: [email protected] Received: 26 August 2020; Accepted: 7 September 2020; Published: 9 September 2020 Abstract: The HIV-1 integrase enzyme (IN) plays a critical role in the viral life cycle by integrating the reverse-transcribed viral DNA into the host chromosome. This function of IN has been well studied, and the knowledge gained has informed the design of small molecule inhibitors that now form key components of antiretroviral therapy regimens. Recent discoveries unveiled that IN has an under-studied yet equally vital second function in human immunodeficiency virus type 1 (HIV-1) replication. This involves IN binding to the viral RNA genome in virions, which is necessary for proper virion maturation and morphogenesis. Inhibition of IN binding to the viral RNA genome results in mislocalization of the viral genome inside the virus particle, and its premature exposure and degradation in target cells. The roles of IN in integration and virion morphogenesis share a number of common elements, including interaction with viral nucleic acids and assembly of higher-order IN multimers. Herein we describe these two functions of IN within the context of the HIV-1 life cycle, how IN binding to the viral genome is coordinated by the major structural protein, Gag, and discuss the value of targeting the second role of IN in virion morphogenesis. Keywords: HIV-1; integrase; maturation; integrase–RNA interactions; protein–RNA interactions 1. -

Clinical Extrapolation of the Effects of Dolutegravir and Other HIV Integrase Inhibitors on Folate Transport Pathways
DMD Fast Forward. Published on June 5, 2019 as DOI: 10.1124/dmd.119.087635 This article has not been copyedited and formatted. The final version may differ from this version. DMD/2019/087635 Clinical Extrapolation of the Effects of Dolutegravir and Other HIV Integrase Inhibitors on Folate Transport Pathways Maciej J. Zamek-Gliszczynski*, Xuexiang Zhang*, Jennypher Mudunuru, Yewei Du, Jian-Lu Chen, Kunal S. Taskar, Jane Huang, Yong Huang, and Elizabeth H. Romach GlaxoSmithKline, Collegeville, PA, USA (MJZ-G, JM), and Ware, UK (KST); BioIVT, Santa Downloaded from Clara, CA, USA (XZ, YD, J-LC, JH, YH); ViiV Healthcare, RTP, NC, USA (EHR) *Authors contributed equally to this work dmd.aspetjournals.org at ASPET Journals on September 28, 2021 1 DMD Fast Forward. Published on June 5, 2019 as DOI: 10.1124/dmd.119.087635 This article has not been copyedited and formatted. The final version may differ from this version. DMD/2019/087635 Running Title: Integrase inhibitor effects on folate transport (48/80 characters) Corresponding Author: Maciej J. Zamek-Gliszczynski, Ph.D. GlaxoSmithKline 1250 South Collegeville Rd Collegeville, PA 19426, USA Downloaded from e-mail: [email protected] Tel: 1-610-270-6278 dmd.aspetjournals.org Number of text pages: 32 at ASPET Journals on September 28, 2021 Number of Figures: 6 Number of Tables: 1 References: 39 Abstract: 250/250 Significance Statement: 118/120 Introduction: 681/750 Discussion: 1,500/1,500 Abbreviations: neural-tube defects (NTDs), proton-coupled folate transporter (PCFT), reduced folate carrier (RFC), folate receptor α (FRα), dihydrofolate reductase (DHFR), Madin-Darby Canine Kidney-II (MDCK-II), inhibitory concentration (IC) 2 DMD Fast Forward.