US Department of Commerce Noaa NATIONAL OCEANIC and ATMOSPHERIC ADMINISTRATION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
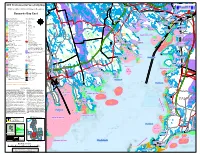
Atlantic Cod5 0 5 D
OND P Y D N S S S S S S S S S S S S S S S S S S S S S S S S S A S S S S S L S P TARKILN HILL O LINCOLN HILL E C G T G ELLIS POND A i S S S S S S S S S S S S S S S S S S S S S S S S S C S S S S Sb S S S S G L b ROBBINS BOG s E B I S r t P o N W o O o n N k NYE BOG Diamondback G y D Þ S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S COWEN CORNER R ! R u e W W S n , d B W "! A W H Þ terrapin W r s D h S S S S S S S S S S S S S S S S S S S S O S S S S S S S S S S S S S S S S S S S S S S S ! S S S S S S S S S S l A N WAREHAM CENTER o e O R , o 5 y k B M P S , "! "! r G E "! Year-round o D DEP Environmental Sensitivity Map P S N ok CAMP N PO S S S S S S S S S S S S S S S S S S S S es H S S S S S S S S S S S S S S S S S S SAR S S S S S S S S S S S S S S O t W SNIPATUIT W ED L B O C 5 ra E n P "! LITTLE c ROGERS BOG h O S S S S S S S S S S S S S S S S S S S S Si N S S S S S S S S S S S S S A S S S S S S S S S S S S S S S S S S BSUTTESRMILKS S p D American lobster G pi A UNION ca W BAY W n DaggerblAaMde grass shrimp POND RI R R VE Þ 4 S S S S S S S S S S S S S S S S S S S S i S S S S S S S S S S S S S S S S S S S S S S S )S S S S S S v + ! "! m er "! SAND la W W ÞÞ WAREHAM DICKS POND Þ POND Alewife c Þ S ! ¡[ ! G ! d S S S S S S S S S S S S S S S h W S S S S S S S S S S S S S S S S S S S 4 S Sr S S S S S S S S ! i BUTTERMILK e _ S b Þ "! a NOAA Sensitive Habitat and Biological Resources q r b "! m ! h u s M ( BANGS BOG a a B BAY n m a Alewife g OAKDALE r t EAST WAREHAM B S S S S S S S S S S S S S -

An Assessment of Polybrominated Diphenyl Ethers (Pbdes) in Sediments and Bivalves of the U.S
An assessment of polybrominated diphenyl ethers (PBDEs) in sediments and bivalves of the U.S. coastal zone Item Type monograph Authors Kimbrough, K.L.; Johnson, W.E.; Lauenstein, G.G.; Christensen, J.D.; Apeti, D.A. Publisher NOAA/National Centers for Coastal Ocean Science Download date 07/10/2021 01:20:08 Link to Item http://hdl.handle.net/1834/30744 NOAA NATIONAL STATUS & TRENDS MUSSEL WATCH PROGRAM An Assessment of Polybrominated Diphenyl Ethers (PBDEs) in Sediments and Bivalves of the U.S. Coastal Zone Mention of trade names or commercial products does not constitute endorsement or recommendation for their use by the United States government. Citation for this Report Kimbrough, K. L., W. E. Johnson, G. G. Lauenstein, J. D. Christensen and D. A. Apeti. 2009. An Assessment of Polybrominated Diphenyl Ethers (PBDEs) in Sediments and Bivalves of the U.S. Coastal Zone. Silver Spring, MD. NOAA Technical Memorandum NOS NCCOS 94. 87 pp. II NOAA National Status & Trends | PBDE Report An Assessment of Polybrominated Diphenyl Ethers (PBDEs) in Sediments and Bivalves of the U.S. Coastal Zone K. L. Kimbrough, W. E. Johnson, G. G. Lauenstein, J. D. Christensen and D. A. Apeti. Center for Coastal Monitoring and Assessment NOAA/NOS/NCCOS 1305 East-West Highway Silver Spring, Maryland 20910 III NOAA National Status & Trends | PBDE Report An Assessment of PBDEs in Sediment and Bivalves of the U.S. Coastal Zone IV NOAA National Status & Trends | PBDE Report An Assessment of PBDEs in Sediment and Bivalves of the U.S. Coastal Zone Background NOAA’s Mussel Watch Program was designed to monitor the status and trends of chemical contamination of U.S. -

NOAA Technical Memorandum NOS OMA 56 STATUS and TRENDS IN
NOAA Technical Memorandum NOS OMA 56 STATUS AND TRENDS IN CONCENTRATIONS OF SELECTED CONTAMINANTS IN BOSTON HARBOR SEDIMENTS AND BIOTA Seattle, Washington June 1991 National Ocean Service Office of Oceanography and Marine Assessment National Ocean Service National Oceanic and Atmospheric Administration U.S. Department of Commerce The Office of Oceanography and Marine Assessment (OMA) provides decisionmakers comprehensive, scientific information on characteristics of the oceans, coastal areas, and estuaries of the United States of America. The information ranges from strategic, national assessments of coastal and estuarine environmental quality to real-time information for navigation or hazardous materials spill response. For example, OMA monitors the rise and fall of water levels at about 200 coastal locations of the USA (including the Great Lakes); predicts the times and heights of high and low tides; and provides information critical to national defense, safe navigation, marine boundary determination, environmental management, and coastal engineering. Currently, OMA is installing the Next Generation Water Level Measurement System that will replace by 1992 existing water level measurement and data processing technologies. Through its National Status and Trends (NS&T) Program, OMA uses uniform techniques to monitor toxic chemical contamination of bottom-feeding fish, mussels and oysters, and sediments at about 300 locations throughout the United States of America. A related NS&T program of directed research examines the relationships between contaminant exposure and indicators of biological responses in fish and shellfish. OMA uses computer-based circulation models and innovative measurement technologies to develop new information products, including real-time circulation data, circulation fore- casts under various meteorological conditions, and circulation data atlases. -

Essential Fish Habitat (EFH) Assessment New Bedford/Fairhaven Harbor Massachusetts March 2002
Essential Fish Habitat (EFH) Assessment New Bedford/Fairhaven Harbor Massachusetts March 2002 Prepared for: Massachusetts Office of Coastal Zone Management 251 Causeway Street, Suite 900 Boston, MA 02114-2119 Prepared by: Maguire Group Inc. 225 Foxborough Boulevard Foxborough, MA 02035 508-543-1700 Table of Contents TABLE OF CONTENTS Section No. Title Page EXECUTIVE SUMMARY ................................................................................................. ES-1 1.0 INTRODUCTION...................................................................................................... 1-1 1.1 Proposed Action ............................................................................................... 1-1 1.2 Purpose and Need............................................................................................. 1-3 1.2.1 Purpose................................................................................................. 1-3 1.2.2 Dredging Need ..................................................................................... 1-3 1.3 Background ...................................................................................................... 1-6 1.3.1 Assessment of Alternative Disposal Sites............................................ 1-6 1.3.2 Identification of the Preferred Alternative ........................................... 1-9 2.0 DESCRIPTION OF THE STUDY AREA ............................................................... 2-1 2.1 Description of the DMMP Disposal Sites....................................................... -

Contaminated Monitoring Report for Seafood Harvested in 2008 from the New Bedford Harbor Superfund Site
Contaminated Monitoring Report for Seafood Harvested in 2008 from the New Bedford Harbor Superfund Site by Massachusetts Department of Environmental Protection and Massachusetts Division of Marine Fisheries July 2010 TABLE of CONTENTS 1. Introduction 2. Seafood Monitoring Program Design 3. 2008 Field Collection 4. Analytical Chemistry 5. Results and Discussion 6. References FIGURES Figure 1 Fish Closure Areas I to III Figure 2 Quahog (Pre and post-spawn) Area II & III Figure 3 Sea Bass Area II & III Figure 4 Alewife Area I Figure 5 Scup Area II & III Figure 6 Bluefish Area II & III Figure 7 PCBs Concentrations in Quahog (Pre-Spawn) Figure 8 PCBs Concentrations in Quahog (Post-Spawn 1) Figure 9 PCBs Concentrations in Quahog (Post-Spawn 2) Figure 10 PCBs Concentrations in Black Sea Bass Figure 11 PCBs Concentrations in Scup TABLES Table 1 Summary of Sample Data for Pre-Spawn Quahog Table 2 Summary of Sample Data for Post-Spawn 1 Quahog Table 3 Summary of Sample Data for Post-Spawn 2 Quahog Table 4 Comparison of Pre-Spawn and Post Spawn Quahog Table 5 Summary of Sample Data for Sea Bass Table 6 Summary of Sample Data for Alewife and Scup Table 7 Summary of Sample Data for Bluefish APPENDICIES Appendix A Laboratory Data Appendix B Data Validation Summary, MassDEP, NBH Seafood Contaminant Survey Monitoring 2008 Sampling Appendix C Seafood Monitoring - Field Sampling Activities for the NBH Superfund Site 2008 Annual Report Appendix D Congeners Used to Quantitate Aroclors / Determination of PCBs by GC/MS-SIM for Aroclor 1. Introduction This report documents the levels of PCBs (polychlorinated biphenyls) measured in edible seafood species caught in New Bedford Harbor and surrounding Buzzards Bay in southeastern Massachusetts in 2008. -

New Bedford, Monitoring Report for Final Seafood
Monitoring Report for Seafood Harvested in 2013 from the New Bedford Harbor Superfund Site by Massachusetts Department of Environmental Protection and Massachusetts Division of Marine Fisheries June 2014 TABLE OF CONTENTS 1. Introduction 2. Seafood Monitoring Program Design 3. 2013 Field Collection 4. Analytical Chemistry 5. Results and Discussion 6. References FIGURES Figure 1 PCB Sample Locations Areas 1 to 3 Figure 2 Alewife Sample Location Area 1 Figure 3 Black Sea Bass Sample Locations Areas 2 & 3 Figure 4 Bluefish Sample Locations Areas 2 & 3 Figure 5 Conch Sample Locations Areas 2 & 3 Figure 6 Quahog (Pre-spawn) Sample Locations Areas 2 to 3 Figure 7 Quahog (Post-Spawn) Sample Locations Areas 2 & 3 Figure 8 Scup Sample Locations Areas 2 & 3 Figure 9 Tautog Sample Locations Areas 2 & 3 Figure 10 Striped Bass Sample Locations Areas 2 & 3 Figure 11 Striped Bass Sample Locations Off-Site Figure 12 PCBs Concentrations in Black Sea Bass Areas 2 & 3 Figure 13 PCBs Concentrations in Bluefish Areas 2 & 3 Figure 14 PCBs Concentrations in Conch Areas 2 & 3 Figure 15 PCBs Concentrations in Quahog (Pre-Spawn) Areas 2 & 3 Figure 16 PCBs Concentrations in Quahog (Post-Spawn) Areas 2 & 3 Figure 17 PCBs Concentrations in Scup Areas 2 & 3 Figure 18 PCBs Concentrations in Tautog Areas 2 & 3 Figure 19 PCBs Concentrations in Striped Bass Areas 2 & 3 Figure 20 PCBs Concentrations in Striped Bass Off-Site TABLES Table 1 Summary of Sample Data for Alewife Area 1 Table 2 Summary of Sample Data for Black Sea Bass Areas 2 & 3 Table 3 Summary of Sample Data -

Method Mwdata
MARINE ENVIRONMENTAL RESEARCH Marine Environmental Research 62 (2006) 261–285 www.elsevier.com/locate/marenvrev Trends in chemical concentrations in mussels and oysters collected along the US coast: Update to 2003 Thomas P. O’Connor 1, Gunnar G. Lauenstein * National Oceanic and Atmospheric Administration, National Center for Coastal Ocean Sciences, Center for Coastal Monitoring and Assessment, NOAA N/SCI1, 1305 East West Highway, Silver Spring, MD 20910, USA Received 14 September 2005; received in revised form 17 February 2006; accepted 25 April 2006 Abstract With data from the annual analyses of mussels and oysters collected in 1986–1993 from sites located throughout the coastal United States [O’Connor, T.P., 1996. Trends in chemical concentrations in mussels and oysters collected along the US coast from 1986 to 1993. Mar. Environ. Res. 41, 183– 200] showed decreasing trends, on a national scale, for chemicals whose use has been banned or has greatly decreased and that concentrations of most other chemicals were neither increasing nor decreas- ing. With data through 2003 those conclusions still apply. National median concentrations of synthetic organic chemicals and cadmium continue to decrease. The added data show that concentrations of lin- dane and high molecular weight PAHs are also decreasing on a national scale. For metals other than cadmium and zinc (in mussels), the added data reveal trends at more sites than in 1993 but no addi- tional national trends. However, the longer time series has revealed several local and regional trends. Published by Elsevier Ltd. Keywords: Mussel Watch; Temporal trends; Chlorinated hydrocarbons; Butyltins; PAHs; Trace elements 1. -

Table 1. Results of the 1994-95 Massachusetts Coastal Colonial
Table 1. Results of the 1994-95 Massachusetts Coastal Colonial Waterbird Inventory showing estimated numbers of pairs for all species at all colonies located in the coastal zone. An asterisk (*) indicates "count not taken." Data compiled by B.G. Blodget, Massachusetts Division of Fisheries and Wildlife. COLONY SPECIES NO. OF PAIRS CENSUS REPORTING NUMBER COLONY NAME, TOWN CODE 1994 1995 METHOD1 AUTHORITY 324007.0 Woodbridge I., Newburyport COTE 65 62 ACG FWS 324007.1 Blackwater R. Group, Salisbury 0 0 TTOR 324007.2 Chaces I., Newbury COTE 14 0 ACG FWS HEGU 0 1 NCG FWS 324008.0 Plum I. R. Group, Newbury COTE 34 30 NCG FWS 324009.0 Parker R. Group, Newbury COTE 10 15 NCG FWS 324009.1 Plum I. Beach, Newbury-Rowley-Ip LETE 32 19 NCG FWS 324010.0 Roger I., Ipswich COTE 6 2 ACB ECG 324010.1 Stage I.-Cross Hill F., Ipswich * 0 FWS 324010.2 Bagwell I., Ipswich COTE 8 8 ACB ECG 324010.3 Rowley Salt Marshes, Rowley COTE 3 2 ACB ECG 324010.4 Lord's I., Ipswich COTE 25 20 ACB ECG 324010.5 Ipswich Salt Marshes, Ipswich COTE 13 13 ACB ECG 324011.0 Crane Beach, Ipswich LETE 30 75 NCG TTOR 324012.0 Tenpound I., Gloucester HEGU 75 * NCG DFW GBBG 70 * NCG DFW 324013.0 Norman's Woe, Gloucester DCCO 199 * NCG DFW HEGU 76 * NCG DFW TABLE 1... 2 COLONY SPECIES NO. OF PAIRS CENSUS REPORTING NUMBER COLONY NAME, TOWN CODE 1994 1995 METHOD AUTHORITY GBBG 4 * NCG DFW 324014.0 Kettle I., Gloucester GREG 42 * NCG DFW SNEG 207 * NCG DFW LBHE 6 * NCG DFW BCNH 7 * NCG DFW GLIB 35 * NCG DFW HEGU 388 * NCG DFW GBBG 146 * NCG DFW 324015.0 Graves I., Manchester HEGU 97 * NCG DFW GBBG 74 * NCG DFW 324016.0 Great Egg Rock, Manchester DCCO 124 * NCB DFW GBBG 2 * NCB DFW 324017.0 Dry Salvages, Rockport DCCO * 19 NCG DFW 324018.0 Straitsmouth I., Rockport HEGU 23 * NCG MAS GBBG 143 * NCG MAS 324019.0 Thacher I., Rockport BCNH 1 1 NCG DFW GLIB 0 1 AEG DFW HEGU 1,185 1,359 NCG FWS TABLE 1.. -

BEHAVIORAL HEALTH PROVIDERS (Effective 1/1/2012) for More Information About Any Providers Listed Below, Please Call Our Member Services Department at 877-492-6967
BMC HEALTHNET PLAN SELECT -- BEHAVIORAL HEALTH PROVIDERS (effective 1/1/2012) For more information about any providers listed below, please call our Member Services department at 877-492-6967. Office Street Facility Name Last Name First Name Address Office Suite City State Zip Office Phone Medical Group Affiliation AdCare Hospital of Worcester, AdCare Hospital of Worcester, Inc. - Worcester Site 107 Lincoln Street Worcester MA 01605 (800)345-3552 Inc. - Worcester Site Arbour Hospital 49 Robinwood Ave Boston MA 02130 (617)522-4400 Arbour Hospital Dimock Detox 55 Dimock Street Roxbury MA 02119 (617)442-9661 Dimock Detox Arbour HRI Hospital 227 Babcock St. Brookline MA 02146 (617)731-3200 Arbour HRI Hospital Child and Family Services, Inc. 1061 Pleasant Street New Bedford MA 02740 (508)996-8572 Child and Family Services, Inc. Child and Family Services, Inc. 1061 Pleasant Street New Bedford MA 02740 (508)996-8572 Child and Family Services, Inc. Bayridge Hospital 60 Granite Street Lynn MA 01904 (781)599-9200 Bayridge Hospital Bournewood Hospital 300 South Street Brookline MA 02467 (617)469-0300 Bournewood Hospital Bournewood Hospital 300 South Street Brookline MA 02467 (617)469-0300 Bournewood Hospital 1493 Cambridge Cambridge Hospital Street Cambridge MA 02139 (617)498-1000 Cambridge Hospital Community Healthlink - 72 Jaques Community Healthlink - 72 Avenue 72 Jaques Avenue Worcester MA 01610 (508)860-1260 Jaques Avenue Faulkner Hospital 1153 Center Street Jamaica Plain MA 02130 (617)983-7711 Faulkner Hospital Lowell Community Health Lowell Community Health Initiative 15-17 Warren Street Lowell MA 01852 (978)937-9448 Initiative McLean Hospital 115 Mill St. -

Summary of 2006 Census of American Oystercatchers in Massachusetts
SUMMARY OF 2006 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS Compiled by: Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 August 2007 SUMMARY OF 2006 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS INTRODUCTION This report summarizes data collected during a statewide census of the American Oystercatcher (Haematopus palliatus) in Massachusetts during the 2006 breeding season. This census was conducted by a statewide network of cooperating agencies and organizations. The American Oystercatcher is a large, strikingly colored shorebird that nests on coastal beaches along the Atlantic Coast from Maine to Florida. Although the species has expanded its range and increased in abundance in New England over the past 50 years, its range extension northward along the Atlantic Coast may well be a recolonization of formerly occupied habitat (Forbush 1912). On a continental scale, the American Oystercatcher is one of the most uncommon species of breeding shorebirds in North America and has been designated a Species of High Concern in the United States Shorebird Conservation Plan (Brown et. al. 2001). METHODS Data on American Oystercatcher abundance, distribution, and reproductive success were collected by a coast-wide group of cooperators that included full-time and seasonal biologists and coastal waterbird monitors, beach managers, researchers, and volunteers. This is the same group that conducts annual censuses of Piping Plovers and terns in Massachusetts (Melvin 2007, Mostello 2007). Observers censused adult oystercatchers during or as close as possible to the designated census period of 22 - 31 May 2006, in order to minimize double-counting of birds that might move between multiple sites during the breeding season. -

Mussel Watch Program
An assessment of two decades of contaminant monitoring in the Nation’s Coastal Zone. Item Type monograph Authors Kimbrough, K. L.; Lauenstein, G. G.; Christensen, J. D.; Apeti, D. A. Publisher NOAA/National Centers for Coastal Ocean Science Download date 08/10/2021 06:22:29 Link to Item http://hdl.handle.net/1834/20041 NOAA NATIONAL STATUS & TRENDS MUSSEL WATCH PROGRAM An Assessment of Two Decades of Contaminant Monitoring in the Nation’s Coastal Zone Mention of trade names or commercial products does not constitute endorsement or recommendation for their use by the United States government. Citation for this Report Kimbrough, K. L., W. E. Johnson, G. G. Lauenstein, J. D. Christensen and D. A. Apeti. 2008. An Assessment of Two Decades of Contaminant Monitoring in the Nation’s Coastal Zone. Silver Spring, MD. NOAA Technical Memorandum NOS NCCOS 74. 105 pp. iiii NOAA National Status & Trends | Mussel Watch Report An Assessment of Two Decades of Contaminant Monitoring in the Nation’s Coastal Zone An Assessment of Two Decades of Contaminant Monitoring in the Nation’s Coastal Zone K. L. Kimbrough, W. E. Johnson, G. G. Lauenstein, J. D. Christensen and D. A. Apeti. National Oceanic and Atmospheric Administration National Ocean Service National Centers for Coastal Ocean Science Center for Coastal Monitoring and Assessment 1305 East-West Highway Silver Spring, Maryland 20910 ii NOAA National Status & Trends | Mussel Watch Report An Assessment of Two Decades of Contaminant Monitoring in the Nation’s Coastal Zone iiii NOAA National Status & Trends -

Table 1), from a Total of 229 Sites Surveyed (Tables 1, 2)
SUMMARY OF 2009 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS Compiled by: Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 July 30, 2010 SUMMARY OF 2009 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS INTRODUCTION This report summarizes data collected during a statewide census of the American Oystercatcher (Haematopus palliatus) in Massachusetts during the 2009 breeding season. This census was conducted by observers affiliated with a statewide network of cooperating agencies and organizations. The American Oystercatcher is a large, strikingly colored shorebird that nests on coastal beaches along the Atlantic Coast from Maine to Florida. Although the species has expanded its range and increased in abundance in New England over the past 50 years, its range extension northward along the Atlantic Coast may well be a recolonization of formerly occupied habitat (Forbush 1912). On a continental scale, the American Oystercatcher is one of the most uncommon species of breeding shorebirds in North America and is designated a Species of High Concern in the United States Shorebird Conservation Plan (Brown et. al. 2001). METHODS Data on American Oystercatcher abundance, distribution, and reproductive success were collected by a coast-wide group of cooperators that included full-time and seasonal biologists and coastal waterbird monitors, beach managers, researchers, and volunteers. This is the same group that conducts annual censuses of Piping Plovers and terns in Massachusetts (Melvin 2010, Mostello 2010). Observers censused adult oystercatchers during or as close as possible to the designated census period of 22 - 31 May 2009, in order to minimize double-counting of birds that might move between multiple sites during the breeding season.