Rho Activation at a Glance Rho Activation at a Glance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytoplasmic Expression of Epithelial Cell Transforming Sequence 2 in Lung Adenocarcinoma and Its Implications for Malignant Progression
Laboratory Investigation (2019) 99:551–567 https://doi.org/10.1038/s41374-018-0142-4 ARTICLE Cytoplasmic expression of epithelial cell transforming sequence 2 in lung adenocarcinoma and its implications for malignant progression 1 2 3 1 2 2 Zeinab Kosibaty ● Yoshihiko Murata ● Yuko Minami ● Tomoko Dai ● Junko Kano ● Ryota Matsuoka ● 2 2 Noriyuki Nakano ● Masayuki Noguchi Received: 8 March 2018 / Revised: 14 August 2018 / Accepted: 20 August 2018 / Published online: 12 December 2018 © United States & Canadian Academy of Pathology 2018 Abstract Epithelial cell transforming sequence 2 (ECT2), a guanine nucleotide exchange factor, is predominantly localized in the nucleus of non-transformed cells and functions to regulate cytokinesis. ECT2 is also localized in the cytoplasm of cancer cells. Aberrant cytoplasmic expression of ECT2 is thought to drive tumor growth and invasion. In this study, we investigated the cytoplasmic expression of ECT2 and its prognostic and biological significance in lung adenocarcinoma. Western blotting of cellular fractions from the nucleus and cytoplasm was performed to determine the subcellular localization of ECT2 in lung adenocarcinoma cell lines. The cytoplasmic expression of ECT2 in 167 lung fi 1234567890();,: 1234567890();,: adenocarcinomas was evaluated by immunohistochemistry and its clinical signi cance was examined using Kaplan–Meier curves and Cox regression analysis. Scraping cytology specimens of 13 fresh lung adenocarcinomas were used to assess the subcellular localization of ECT2 and its phosphorylation at Thr790 (P-ECT2(T790)). We found that ECT2 was localized in both the nucleus and cytoplasm of lung adenocarcinoma cell lines and tumor tissues. Cytoplasmic expression of ECT2 was detected by immunohistochemistry in 83 (50%) of the lung adenocarcinomas, and was found to increase during cancer progression. -
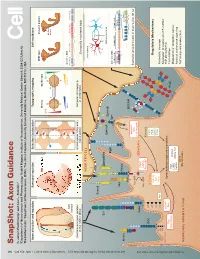
Snapshot: Axon Guidance Pasterkamp R
494 1 Cell Cell ??? SnapShot: Axon Guidance 153 SnapShot: XXXXXXXXXXXXXXXXXXXXXXXXXX 1 2 , ??MONTH?? ??DATE??, 200? ©200? Elsevier Inc. 200?©200? ElsevierInc. , ??MONTH?? ??DATE??, DOI R. Jeroen Pasterkamp and Alex L. Kolodkin , April11, 2013©2013Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.03.031 AUTHOR XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX 1 AFFILIATIONDepartment of XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Neuroscience and Pharmacology, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, 3584 CG Utrecht, The Netherlands; 2Department of Neuroscience, HHMI, The Johns Hopkins University School of Medicine, Baltimore, MD 21212, USA Axon attraction and repulsion Surround repulsion Selective fasciculation Topographic mapping Self-avoidance Wild-type Dscam1 mutant Sema3A A Retina SC/Tectum EphB EphrinB P D D Mutant T A neuron N P A V V Genomic DNA P EphA EphrinA Slit Exon 4 (12) Exon 6 (48) Exon 9 (33) Exon 17 (2) Netrin Commissural axon guidance Surround repulsion of peripheral Grasshopper CNS axon Retinotectal mapping at the CNS midline nerves in vertebrates fasciculation in vertebrates Drosophila mushroom body XXXXXXXXX NEURITE/CELL Isoneuronal Sema3 Slit Sema1/4-6 Heteroneuronal EphrinA EphrinB FasII Eph Genomic DNA Pcdh-α (14) Pcdh-β (22) Pcdh-γ (22) Variable Con Variable Con Nrp Plexin ** *** Netrin LAMELLIPODIA Con DSCAM ephexin Starburst amacrine cells in mammalian retina Ras-GTP Vav See online version for legend and references. α-chimaerin GEFs/GAPs Robo FARP Ras-GDP LARG RhoGEF Kinases DCC cc0 GTPases PKA cc1 FAK Regulatory Mechanisms See online versionfor??????. Cdc42 GSK3 cc2 Rac PI3K P1 Rho P2 cc3 Abl Proteolytic cleavage P3 Regulation of expression (TF, miRNA, FILOPODIA srGAP Cytoskeleton regulatory proteins multiple isoforms) Sos Trio Pcdh Cis inhibition DOCK180 PAK ROCK Modulation of receptors’ output LIMK Myosin-II Colin Forward and reverse signaling Actin Trafcking and endocytosis NEURONAL GROWTH CONE Microtubules SnapShot: Axon Guidance R. -

Cell Division Symmetry Control and Cancer Stem Cells
AIMS Molecular Science, 7(2): 82–98. DOI: 10.3934/molsci.2020006 Received: 15 February 2020 Accepted: 26 April 2020 Published: 06 May 2020 http://www.aimspress.com/journal/Molecular Review Cell division symmetry control and cancer stem cells Sreemita Majumdar and Song-Tao Liu* Department of Biological Sciences, University of Toledo, Toledo, OH 43606, USA * Correspondence: Email: [email protected]; Tel: +14195307853. Table S1. Genes encoding polarity and fate-determinant proteins involved in asymmetric cell division. C. elegans1 D. melanogaster 1 Mammals1 Description2 Associated with/ Interactors 3 Cellular Localization (mammalian cell)4 Serine/threonine protein microtubule-associated protein cell membrane, peripheral and lateral, par-1 par-1 MARK1/2/3/4 kinase MAPT/TAU cytoplasm, dendrite RING, Lipid binding par-2 - - domain PDZ for membrane, cell junction, adherens junction, cell cortex, par-3 baz PARD3 Oligomerization domain at actin, PARD6 endomembrane system, NTD Continued on next page 2 C. elegans1 D. melanogaster 1 Mammals1 Description2 Associated with/ Interactors 3 Cellular Localization (mammalian cell)4 Serine/threonine-protein nucleus, mitochondria, cytoplasm, par-4 Lkb1 STK11/LKB1 STRAD complex kinase membrane 14-3-3 domain binding par-5 14-3-3 YWHAB phosphoserine/ adapter to many proteins cytoplasm phosphothreonine motif cell membrane, centriolar satellite, actin par-6 par-6 PARD6A/B/G PB1, CRIB, PDZ PARD3 cytoskeleton,centrosome, cytoplasm ,ruffles PARD3, and a PARD6 protein PB1, AGC-Kinase (PARD6A, PARD6B or PARD6G) pkc-3 aPKC PRKCI/Z domain, DAG binding, cytoplasm, nucleus, membrane and a GTPase protein (CDC42 or Zinc finger domain RAC1), LLGL1,ECT2 LRR and PDZ protein Cadherin, Scrib-APC-beta-catenin nucleoplasm, basolateral plasma membrane, let-413 scrib SCRIB family. -

The Rac Gtpase in Cancer: from Old Concepts to New Paradigms Marcelo G
Published OnlineFirst August 14, 2017; DOI: 10.1158/0008-5472.CAN-17-1456 Cancer Review Research The Rac GTPase in Cancer: From Old Concepts to New Paradigms Marcelo G. Kazanietz1 and Maria J. Caloca2 Abstract Rho family GTPases are critical regulators of cellular func- mislocalization of Rac signaling components. The unexpected tions that play important roles in cancer progression. Aberrant pro-oncogenic functions of Rac GTPase-activating proteins also activity of Rho small G-proteins, particularly Rac1 and their challenged the dogma that these negative Rac regulators solely regulators, is a hallmark of cancer and contributes to the act as tumor suppressors. The potential contribution of Rac tumorigenic and metastatic phenotypes of cancer cells. This hyperactivation to resistance to anticancer agents, including review examines the multiple mechanisms leading to Rac1 targeted therapies, as well as to the suppression of antitumor hyperactivation, particularly focusing on emerging paradigms immune response, highlights the critical need to develop ther- that involve gain-of-function mutations in Rac and guanine apeutic strategies to target the Rac pathway in a clinical setting. nucleotide exchange factors, defects in Rac1 degradation, and Cancer Res; 77(20); 5445–51. Ó2017 AACR. Introduction directed toward targeting Rho-regulated pathways for battling cancer. Exactly 25 years ago, two seminal papers by Alan Hall and Nearly all Rho GTPases act as molecular switches that cycle colleagues illuminated us with one of the most influential dis- between GDP-bound (inactive) and GTP-bound (active) forms. coveries in cancer signaling: the association of Ras-related small Activation is promoted by guanine nucleotide exchange factors GTPases of the Rho family with actin cytoskeleton reorganization (GEF) responsible for GDP dissociation, a process that normally (1, 2). -

Regulation of the Mammalian Target of Rapamycin Complex 2 (Mtorc2)
Regulation of the Mammalian Target Of Rapamycin Complex 2 (mTORC2) Inauguraldissertation Zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Klaus-Dieter Molle aus Heilbronn, Deutschland Basel, 2006 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät Auf Antrag von Prof. Michael N. Hall und Prof. Markus Affolter. Basel, den 21.11.2006 Prof. Hans-Peter Hauri Dekan Summary The growth controlling mammalian Target of Rapamycin (mTOR) is a conserved Ser/Thr kinase found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. The tumor suppressor TSC1-TSC2 complex inhibits mTORC1 by acting on the small GTPase Rheb, but the role of TSC1-TSC2 and Rheb in the regulation of mTORC2 is unclear. Here we examined the role of TSC1-TSC2 in the regulation of mTORC2 in human embryonic kidney 293 cells. Induced knockdown of TSC1 and TSC2 (TSC1/2) stimulated mTORC2-dependent actin cytoskeleton organization and Paxillin phosphorylation. Furthermore, TSC1/2 siRNA increased mTORC2-dependent Ser473 phosphorylation of plasma membrane bound, myristoylated Akt/PKB. This suggests that loss of Akt/PKB Ser473 phosphorylation in TSC mutant cells, as reported previously, is due to inhibition of Akt/PKB localization rather than inhibition of mTORC2 activity. Amino acids and overexpression of Rheb failed to stimulate mTORC2 signaling. Thus, TSC1-TSC2 also inhibits mTORC2, but possibly independently of Rheb. Our results suggest that mTORC2 hyperactivation may contribute to the pathophysiology of diseases such as cancer and Tuberous Sclerosis Complex. i Acknowledgement During my PhD studies in the Biozentrum I received a lot of support from many people around me who I mention here to express my gratefulness. -

The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination
The Journal of Neuroscience, August 31, 2011 • 31(35):12579–12592 • 12579 Cellular/Molecular The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination Junji Yamauchi,1,3,5 Yuki Miyamoto,1 Hajime Hamasaki,1,3 Atsushi Sanbe,1 Shinji Kusakawa,1 Akane Nakamura,2 Hideki Tsumura,2 Masahiro Maeda,4 Noriko Nemoto,6 Katsumasa Kawahara,5 Tomohiro Torii,1 and Akito Tanoue1 1Department of Pharmacology and 2Laboratory Animal Resource Facility, National Research Institute for Child Health and Development, Setagaya, Tokyo 157-8535, Japan, 3Department of Biological Sciences, Tokyo Institute of Technology, Midori, Yokohama 226-8501, Japan, 4IBL, Ltd., Fujioka, Gumma 375-0005, Japan, and 5Department of Physiology and 6Bioimaging Research Center, Kitasato University School of Medicine, Sagamihara, Kanagawa 252-0374, Japan In development of the peripheral nervous system, Schwann cells proliferate, migrate, and ultimately differentiate to form myelin sheath. In all of the myelination stages, Schwann cells continuously undergo morphological changes; however, little is known about their underlying molecular mechanisms. We previously cloned the dock7 gene encoding the atypical Rho family guanine-nucleotide exchange factor (GEF) and reported the positive role of Dock7, the target Rho GTPases Rac/Cdc42, and the downstream c-Jun N-terminal kinase in Schwann cell migration (Yamauchi et al., 2008). We investigated the role of Dock7 in Schwann cell differentiation and myelination. Knockdown of Dock7 by the specific small interfering (si)RNA in primary Schwann cells promotes dibutyryl cAMP-induced morpholog- ical differentiation, indicating the negative role of Dock7 in Schwann cell differentiation. It also results in a shorter duration of activation of Rac/Cdc42 and JNK, which is the negative regulator of myelination, and the earlier activation of Rho and Rho-kinase, which is the positive regulator of myelination. -

Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding Rnas
Review Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding RNAs Yu Zhang †, Jun Li †, Xing-Ning Lai, Xue-Qiao Jiao, Jun-Ping Xiong and Li-Xia Xiong * Department of Pathophysiology, Jiangxi Province Key Laboratory of Tumor Pathogenesis and Molecular Pathology, Medical College, Nanchang University, 461 Bayi Road, Nanchang 330006, China; [email protected] (Y.Z.); [email protected] (J.L.); [email protected] (X.-N.L.); [email protected] (X.-Q.J.); [email protected] (J.-P.X.) * Correspondence: [email protected]; Tel.: +86-791-8636-0556 † These authors contributed equally to this work. Received: 30 December 2018; Accepted: 8 February 2019; Published: 11 February 2019 Abstract: Breast cancer is the most common malignant tumors in females. Although the conventional treatment has demonstrated a certain effect, some limitations still exist. The Rho guanosine triphosphatase (GTPase) Cdc42 (Cell division control protein 42 homolog) is often upregulated by some cell surface receptors and oncogenes in breast cancer. Cdc42 switches from inactive guanosine diphosphate (GDP)-bound to active GTP-bound though guanine-nucleotide- exchange factors (GEFs), results in activation of signaling cascades that regulate various cellular processes such as cytoskeletal changes, proliferation and polarity establishment. Targeting Cdc42 also provides a strategy for precise breast cancer therapy. In addition, Cdc42 is a potential target for several types of non-coding RNAs including microRNAs and lncRNAs. These non-coding RNAs is extensively involved in Cdc42-induced tumor processes, while many of them are aberrantly expressed. Here, we focus on the role of Cdc42 in cell morphogenesis, proliferation, motility, angiogenesis and survival, introduce the Cdc42-targeted non-coding RNAs, as well as present current development of effective Cdc42-targeted inhibitors in breast cancer. -

Small Rho Gtpase Family Member Cdc42 and Its Role in Neuronal Survival and Apoptosis
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 1-1-2017 Small Rho GTPase Family Member Cdc42 and Its Role in Neuronal Survival and Apoptosis Noelle Christine Punessen University of Denver Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Biology Commons, and the Genetics and Genomics Commons Recommended Citation Punessen, Noelle Christine, "Small Rho GTPase Family Member Cdc42 and Its Role in Neuronal Survival and Apoptosis" (2017). Electronic Theses and Dissertations. 1337. https://digitalcommons.du.edu/etd/1337 This Thesis is brought to you for free and open access by the Graduate Studies at Digital Commons @ DU. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. Small Rho GTPase Family Member Cdc42 and its Role in Neuronal Survival and Apoptosis A Thesis Presented to the Faculty of Natural Sciences and Mathematics University of Denver In Partial Fulfillment of the Requirements for the Degree Master of Science by Noelle C. Punessen August 2017 Advisor: Dr. Daniel A. Linseman Author: Noelle C. Punessen Title: Small Rho GTPase Family Member Cdc42 and its Role in Neuronal Survival and Apoptosis Advisor: Dr. Daniel A. Linseman Degree Date: August 2017 Abstract Neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer’s and Parkinson’s disease are caused by a progressive and aberrant destruction of neurons in the brain and spinal cord. These disorders lack effective long term treatments, and existing options focus primarily on either delaying disease onset or alleviating symptomology. -

Supplementary Information Method CLEAR-CLIP. Mouse Keratinocytes
Supplementary Information Method CLEAR-CLIP. Mouse keratinocytes of the designated genotype were maintained in E-low calcium medium. Inducible cells were treated with 3 ug/ml final concentration doxycycline for 24 hours before performing CLEAR-CLIP. One 15cm dish of confluent cells was used per sample. Cells were washed once with cold PBS. 10mls of cold PBS was then added and cells were irradiated with 300mJ/cm2 UVC (254nM wavelength). Cells were then scraped from the plates in cold PBS and pelleted by centrifugation at 1,000g for 2 minutes. Pellets were frozen at -80oC until needed. Cells were then lysed on ice with occasional vortexing in 1ml of lysis buffer (50mM Tris-HCl pH 7.4, 100mM NaCl, 1mM MgCl2, 0.1 mM CaCl2, 1% NP-40, 0.5% Sodium Deoxycholate, 0.1% SDS) containing 1X protease inhibitors (Roche #88665) and RNaseOUT (Invitrogen #10777019) at 4ul/ml final concentration. Next, TurboDNase (Invitrogen #AM2238, 10U), RNase A (0.13ug) and RNase T1 (0.13U) were added and samples were incubated at 37oC for 5 minutes with occasional mixing. Samples were immediately placed on ice and then centrifuged at 16,160g at 4oC for 20 minutes to clear lysate. 25ul of Protein-G Dynabeads (Invitrogen #10004D) were used per IP. Dynabeads were pre-washed with lysis buffer and pre- incubated with 3ul of Wako Anti-Mouse-Ago2 (2D4) antibody. The dynabead/antibody mixture was added to the lysate and rocked for 2 hours at 4oC. All steps after the IP were done on bead until samples were loaded into the polyacrylamide gel. -

G Protein Regulation of MAPK Networks
Oncogene (2007) 26, 3122–3142 & 2007 Nature Publishing Group All rights reserved 0950-9232/07 $30.00 www.nature.com/onc REVIEW G Protein regulation of MAPK networks ZG Goldsmith and DN Dhanasekaran Fels Institute for Cancer Research and Molecular Biology, Temple University School of Medicine, Philadelphia, PA, USA G proteins provide signal-coupling mechanisms to hepta- the a-subunits has been used as a basis for the helical cell surface receptors and are criticallyinvolved classification of G proteins into Gs,Gi,Gq and G12 in the regulation of different mitogen-activated protein families in which the a-subunits that show more than kinase (MAPK) networks. The four classes of G proteins, 50% homology are grouped together (Simon et al., defined bythe G s,Gi,Gq and G12 families, regulate 1991). In G-protein-coupled receptor (GPCR)-mediated ERK1/2, JNK, p38MAPK, ERK5 and ERK6 modules by signaling pathways, ligand-activated receptors catalyse different mechanisms. The a- as well as bc-subunits are the exchange of the bound GDP to GTP in the a-subunit involved in the regulation of these MAPK modules in a following which the GTP-bound a-subunit disassociate context-specific manner. While the a- and bc-subunits from the receptor as well as the bg-subunit. The GTP- primarilyregulate the MAPK pathwaysvia their respec- bound a-subunit and the bg-subunit stimulate distinct tive effector-mediated signaling pathways, recent studies downstream effectors including enzymes, ion channels have unraveled several novel signaling intermediates and small GTPase, thus regulating multiple signaling including receptor tyrosine kinases and small GTPases pathways including those involved in the activation of through which these G-protein subunits positivelyas well mitogen-activated protein kinase (MAPK) modules as negativelyregulate specific MAPK modules. -

Cldn19 Clic2 Clmp Cln3
NewbornDx™ Advanced Sequencing Evaluation When time to diagnosis matters, the NewbornDx™ Advanced Sequencing Evaluation from Athena Diagnostics delivers rapid, 5- to 7-day results on a targeted 1,722-genes. A2ML1 ALAD ATM CAV1 CLDN19 CTNS DOCK7 ETFB FOXC2 GLUL HOXC13 JAK3 AAAS ALAS2 ATP1A2 CBL CLIC2 CTRC DOCK8 ETFDH FOXE1 GLYCTK HOXD13 JUP AARS2 ALDH18A1 ATP1A3 CBS CLMP CTSA DOK7 ETHE1 FOXE3 GM2A HPD KANK1 AASS ALDH1A2 ATP2B3 CC2D2A CLN3 CTSD DOLK EVC FOXF1 GMPPA HPGD K ANSL1 ABAT ALDH3A2 ATP5A1 CCDC103 CLN5 CTSK DPAGT1 EVC2 FOXG1 GMPPB HPRT1 KAT6B ABCA12 ALDH4A1 ATP5E CCDC114 CLN6 CUBN DPM1 EXOC4 FOXH1 GNA11 HPSE2 KCNA2 ABCA3 ALDH5A1 ATP6AP2 CCDC151 CLN8 CUL4B DPM2 EXOSC3 FOXI1 GNAI3 HRAS KCNB1 ABCA4 ALDH7A1 ATP6V0A2 CCDC22 CLP1 CUL7 DPM3 EXPH5 FOXL2 GNAO1 HSD17B10 KCND2 ABCB11 ALDOA ATP6V1B1 CCDC39 CLPB CXCR4 DPP6 EYA1 FOXP1 GNAS HSD17B4 KCNE1 ABCB4 ALDOB ATP7A CCDC40 CLPP CYB5R3 DPYD EZH2 FOXP2 GNE HSD3B2 KCNE2 ABCB6 ALG1 ATP8A2 CCDC65 CNNM2 CYC1 DPYS F10 FOXP3 GNMT HSD3B7 KCNH2 ABCB7 ALG11 ATP8B1 CCDC78 CNTN1 CYP11B1 DRC1 F11 FOXRED1 GNPAT HSPD1 KCNH5 ABCC2 ALG12 ATPAF2 CCDC8 CNTNAP1 CYP11B2 DSC2 F13A1 FRAS1 GNPTAB HSPG2 KCNJ10 ABCC8 ALG13 ATR CCDC88C CNTNAP2 CYP17A1 DSG1 F13B FREM1 GNPTG HUWE1 KCNJ11 ABCC9 ALG14 ATRX CCND2 COA5 CYP1B1 DSP F2 FREM2 GNS HYDIN KCNJ13 ABCD3 ALG2 AUH CCNO COG1 CYP24A1 DST F5 FRMD7 GORAB HYLS1 KCNJ2 ABCD4 ALG3 B3GALNT2 CCS COG4 CYP26C1 DSTYK F7 FTCD GP1BA IBA57 KCNJ5 ABHD5 ALG6 B3GAT3 CCT5 COG5 CYP27A1 DTNA F8 FTO GP1BB ICK KCNJ8 ACAD8 ALG8 B3GLCT CD151 COG6 CYP27B1 DUOX2 F9 FUCA1 GP6 ICOS KCNK3 ACAD9 ALG9 -

CDC42 and Three Newly Identified Genes Including the Ras-Related
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 9976-9980, December 1989 Genetics Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSRI (cell polarity/cell cycle/morphogenesis/Saccharomyces cerevisiae/guanine nucleotide-binding protein) ALAN BENDER AND JOHN R. PRINGLE Department of Biology, The University of Michigan, Ann Arbor, MI 48109 Communicated by Leland Hartwell, July 31, 1989 ABSTRACT Genes CDC24, CDC42, and CDC43 are re- Johnson, and J.R.P., unpublished data), and overproduction quired for the establishment ofcell polarity and the localization of the CDC42 product can produce a mislocalization of of secretion in Saccharomyces cerevisiae; mutants defective in budding sites like that seen in some cdc24 mutants (D. these genes fail to form buds and display isotropic expansion of Johnson and J.R.P., unpublished data). Sequencing of the cell surface. To identify other genes that may be involved CDC42 (D. Johnson and J.R.P., unpublished data) revealed in these processes, we screened yeast genomic DNA libraries for that it is a member of the rho family (11) of ras oncogene- heterologous genes that, when overexpressed from a plasmid, related genes and encodes typical domains for GTP binding can suppress a temperature-sensitive cdc24 mutation. We and hydrolysis. Moreover, its C-terminal sequence suggests identified four such genes. One of these proved to be CDC42, that the CDC42 product, like the ras products, may be which has previously been shown to be a member of the rho modified and thence membrane-associated. The available (ras-homologous) family of genes, and a second is a newly observations suggest a tentative model in which the products identified ras-related gene that we named RSR1.