Resting-State Network Mapping in Neurosurgical Practice: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring the Sensorimotor Network Using Functional Connectivity and Graph Theory
EXPLORING THE SENSORIMOTOR NETWORK USING FUNCTIONAL CONNECTIVITY AND GRAPH THEORY by Ronald Bishop Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia August 2014 © Copyright by Ronald Bishop, 2014 TABLE OF CONTENTS LIST OF TABLES ......................................................................................................................................... v LIST OF FIGURES ...................................................................................................................................... vi ABSTRACT ................................................................................................................................................. vii LIST OF ABBREVIATIONS USED ......................................................................................................... viii ACKNOWLEDGEMENTS ......................................................................................................................... xi CHAPTER 1: INTRODUCTION ................................................................................................................. 1 CHAPTER 2: LITERATURE REVIEW ..................................................................................................... 4 2.1 AN OVERVIEW OF NEURONAL COMMUNICATION ..................................................................................... 4 2.2 SYNCHRONOUS NEURAL FIRING .............................................................................................................. -
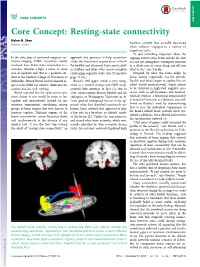
Core Concept: Resting-State Connectivity Helen H
CORE CONCEPTS CORE CONCEPTS Core Concept: Resting-state connectivity Helen H. Shen baseline activity that actually decreased Science Writer when subjects engaged in a variety of cognitive tasks. “It said something important about the In the early days of functional magnetic res- approach that promises to help researchers ongoing activity of the brain, and the fact that onance imaging (fMRI), researchers mostly study the functional organization of both it is not just sitting there waiting for someone analyzed how brain areas responded to a the healthy and abnormal brain, particularly in a white coat to come along and tell you stimulus, whether a light, a noise, or some in children and others who cannot complete what to do,” says Raichle. sort of cognitive task. But as a graduate stu- challenging cognitive tasks. (See Perspective Intrigued by what the brain might be dent at the Medical College of Wisconsin in page 14105.) doing during supposedly inactive periods, Milwaukee, Bharat Biswal had an unusual re- Biswal’s 1995 paper, which is now recog- Raichle and others began to explore this so- quest of his fMRI test subjects: climb into the nized as a seminal resting-state fMRI study, called “default mode network,” which seemed scanner and do, well, nothing. received little attention at first (1). But in to be involved in high-level cognitive pro- Biswal expected that the spontaneous neu- 2001, neuroscientist Marcus Raichle and his cesses, such as self-awareness and memory. ronal chatter at rest would be more or less colleagues at Washington University in St. Michael Greicius, a behavioral neuroscientist random and unstructured. -

Quantitative Mapping of the Brain's Structural Connectivity Using
Quantitative mapping of the brain’s structural connectivity using diffusion MRI tractography: a review Fan Zhanga, Alessandro Daduccib, Yong Hec,d,e,f, Simona Schiavib, Caio Seguing,h, Robert Smithi,j, Chun-Hung Yehk, Tengda Zhaoc,d,e, Lauren J. O’Donnella aBrigham and Women’s Hospital, Harvard Medical School, Boston, USA bDepartment of Computer Science, University of Verona, Verona, Italy cState Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China dBeijing Key Laboratory of Brain Imaging and Connectomics, Beijing Normal University, Beijing, China eIDG/McGovern Institute for Brain Research, Beijing Normal University, Beijing, China fChinese Institute for Brain Research, Beijing, China gMelbourne Neuropsychiatry Centre, University of Melbourne and Melbourne Health, Melbourne, Australia hThe University of Sydney, School of Biomedical Engineering, Sydney, Australia iThe Florey Institute of Neuroscience and Mental Health, Melbourne, Australia jThe University of Melbourne, Melbourne, Australia kInstitute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital, Taoyuan, Taiwan Abstract Diffusion magnetic resonance imaging (dMRI) tractography is an advanced imaging technique that enables in vivo mapping of the brain’s white matter connections at macro scale. Over the last two decades, the study of brain connectivity using dMRI tractography has played a prominent role in the neuroimaging research landscape. In this paper, we provide a high-level overview of how tractography is used to enable quantitative analysis of the brain’s structural connectivity in health and disease. We first provide a review of methodology involved in three main processing steps that are common across most approaches for quantitative analysis of tractography, including methods for tractography correction, segmentation and quantification. -

Resting-State Fmri Reveals Network Disintegration During Delirium T ⁎ Simone J.T
NeuroImage: Clinical 20 (2018) 35–41 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Resting-state fMRI reveals network disintegration during delirium T ⁎ Simone J.T. van Montforta, , Edwin van Dellenb,c, Aletta M.R. van den Boscha,d, Willem M. Ottee,f, Maya J.L. Schutteb, Soo-Hee Choig, Tae-Sub Chungh, Sunghyon Kyeongi, Arjen J.C. Slootera, ⁎⁎ Jae-Jin Kimi, a Department of Intensive Care Medicine, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, The Netherlands b Department of Psychiatry, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, the Netherlands c Melbourne Neuropsychiatry Center, Department of Psychiatry, University of Melbourne, Australia d Faculty of Science, University of Amsterdam, the Netherlands e Biomedical MR Imaging and Spectroscopy Group, Center for Image Sciences, University Medical Center Utrecht, Utrecht University, the Netherlands f Department of Pediatric Neurology, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, the Netherlands g Department of Psychiatry, Institute of Human Behavioral Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea h Department of Radiology, Yonsei University Gangnam Severance Hospital, Seoul, Republic of Korea i Department of Psychiatry, Institute of Behavioral Science in Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea. ARTICLE INFO ABSTRACT Keywords: Delirium is characterized by inattention and other cognitive deficits, symptoms that have been associated with Delirium disturbed interactions between remote brain regions. Recent EEG studies confirm that disturbed global network fMRI topology may underlie the syndrome, but lack an anatomical basis. The aim of this study was to increase our Resting-state understanding of the global organization of functional connectivity during delirium and to localize possible Brain networks alterations. -

Manual for Clinical Language Tractography
Acta Neurochirurgica https://doi.org/10.1007/s00701-019-03899-0 TECHNICAL NOTE - NEUROSURGICAL ANATOMY Manual for clinical language tractography Lucius Fekonja1,2 & Ziqian Wang1 & Ina Bährend1 & Tizian Rosenstock1 & Judith Rösler1 & Lara Wallmeroth1 & Peter Vajkoczy1 & Thomas Picht1,2 Received: 18 January 2019 /Accepted: 28 March 2019 # The Author(s) 2019 Abstract Background We introduce a user-friendly, standardized protocol for tractography of the major language fiber bundles. Method The introduced method uses dMRI images for tractography whereas the ROI definition is based on structural T1 MPRAGE MRI templates, without normalization to MNI space. ROIs for five language-relevant fiber bundles were visualized on an axial, coronal, or sagittal view of T1 MPRAGE images. The ROIs were defined based upon the tracts’ obligatory pathways, derived from literature and own experiences in peritumoral tractography. Results The resulting guideline was evaluated for each fiber bundle in ten healthy subjects and ten patients by one expert and three raters. Overall, 300 ROIs were evaluated and compared. The targeted language fiber bundles could be tracked in 88% of the ROI pairs, based on the raters’ result blinded ROI placements. The evaluation indicated that the precision of the ROIs did not relate to the varying experience of the raters. Conclusions Our guideline introduces a standardized language tractography method for routine preoperative workup and for research contexts. The ROI placement guideline based on easy-to-identify anatomical landmarks -

Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes
JCN Open Access ORIGINAL ARTICLE pISSN 1738-6586 / eISSN 2005-5013 / J Clin Neurol 2019;15(2):211-220 / https://doi.org/10.3988/jcn.2019.15.2.211 Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes Hyun-Soo Choia* Background and Purpose We aimed to reveal resting-state functional connectivity char- Yoon Gi Chungb* c acteristics based on the spike-free waking electroencephalogram (EEG) of benign epilepsy Sun Ah Choi with centrotemporal spikes (BECTS) patients, which usually appears normal in routine visual d Soyeon Ahn inspection. c Hunmin Kim Methods Thirty BECTS patients and 30 disease-free and age- and sex-matched controls Sungroh Yoona were included. Eight-second EEG epochs without artifacts were sampled and then bandpass Hee Hwangc filtered into the delta, theta, lower alpha, upper alpha, and beta bands to construct the associa- Ki Joong Kime,f tion matrix. The weighted phase lag index (wPLI) was used as an association measure for EEG signals. The band-specific connectivity, which was represented as a matrix of wPLI values of a Department of Electrical and all edges, was compared for analyzing the connectivity itself. The global wPLI, characteristic Computer Engineering, path length (CPL), and mean clustering coefficient were compared. Seoul National University, Seoul, Korea Results The resting-state functional connectivity itself and the network topology differed in bHealthcare ICT Research Center, the BECTS patients. For the lower-alpha-band and beta-band connectivity, edges that showed Seoul National University significant differences had consistently lower wPLI values compared to the disease-free con- Bundang Hospital, Seongnam, Korea c trols. -

Comparison of Multiple Tractography Methods for Reconstruction of the Retinogeniculate Visual Pathway Using Diffusion MRI
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.19.304758; this version posted September 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Comparison of multiple tractography methods for reconstruction of the retinogeniculate visual pathway using diffusion MRI Jianzhong He1,2, *, Fan Zhang 2,* ,&, Guoqiang Xie 2,3, Shun Yao4,7 , Yuanjing Feng1,& , Dhiego C. A. Bastos 4, Yogesh Rathi2,5 , Nikos Makris 5,6, Ron Kikinis2 , Alexandra J. Golby 2,4, Lauren J. O’Donnell2 1 Institution of Information Processing and Automation, Zhejiang University of Technology, Hangzhou, China 2 Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 3 Department of Neurosurgery, Nuclear Industry 215 Hospital of Shaanxi Province, Xianyang, China 4 Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 5 Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 6 Departments of Psychiatry, Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA 7 Center for Pituitary Tumor Surgery, Department of Neurosurgery, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China * Co-first-authors; & Co-corresponding-authors 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.19.304758; this version posted September 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract The retinogeniculate visual pathway (RGVP) conveys visual information from the retina to the lateral geniculate nucleus. -

White Matter Tractography and Diffusion&Hyphen;Weighted Imaging
White Matter Advanced article Article Contents Tractography and • Introduction • DWI Acquisition and Analysis Diffusion-weighted • Application • Pitfalls and Limitations Imaging • Conclusion st Jean M Vettel, U.S. Army Research Laboratory, Aberdeen, Maryland, USA Online posting date: 31 October 2017 Nicole Cooper, U.S. Army Research Laboratory, Aberdeen, Maryland, USA Javier O Garcia, U.S. Army Research Laboratory, Aberdeen, Maryland, USA Fang-Cheng Yeh, University of Pittsburgh, Pittsburgh, Pennsylvania, USA Timothy D Verstynen, Carnegie Mellon University, Pittsburgh, Pennsylvania, USA Human cognition requires coordinated commu- to a massively interconnected network (Azevedo et al., 2009). nication across macroscopic brain networks. This network is composed of both gray matter (cell bodies) and This coordination is fundamentally constrained white matter (axons), which together enable the brain’s ability by how populations of neurons are connected to decode, store and send information in support of human cog- nition and behaviour (Passingham et al., 2002). Consequently, together. Understanding how structural connec- coordinated communication across the brain is fundamentally tivity between brain regions constrains or predicts constrained by patterns of interconnections and networks of spe- variability within and between individuals is a cialised processing. pervasive topic of cutting edge research in neu- Gray matter is imaged and studied to understand the com- roscience and the focus of multimillion dollar putational processing or neural -

Neuroimage: Clinical 15 (2017) 513–524
NeuroImage: Clinical 15 (2017) 513–524 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Disruption to control network function correlates with altered dynamic MARK connectivity in the wider autism spectrum ⁎ N. de Lacya,b, D. Dohertyb,c, B.H. Kingd, S. Rachakondae, V.D. Calhoune,f, a Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195, USA b Seattle Children's Research Institute, Center for Integrative Brain Research, Seattle, WA 98105, USA c Department of Pediatrics, Divisions of Developmental and Genetic Medicine, University of Washington, Seattle, WA 98195, USA d Department of Psychiatry, University of California San Francisco, San Francisco, CA 94143, USA e The Mind Research Network & LBERI, Albuquerque, NM 87106, USA f Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM 87131, USA ARTICLE INFO ABSTRACT Keywords: Autism is a common developmental condition with a wide, variable range of co-occurring neuropsychiatric Autism symptoms. Contrasting with most extant studies, we explored whole-brain functional organization at multiple Functional MRI levels simultaneously in a large subject group reflecting autism's clinical diversity, and present the first network- Dynamic connectivity based analysis of transient brain states, or dynamic connectivity, in autism. Disruption to inter-network and inter- Control networks system connectivity, rather than within individual networks, predominated. We identified coupling disruption in Task-positive the anterior-posterior default mode axis, and among specific control networks specialized for task start cues and Default-mode the maintenance of domain-independent task positive status, specifically between the right fronto-parietal and cingulo-opercular networks and default mode network subsystems. -

Development of Thalamocortical Connectivity During Infancy and Its Cognitive Correlations
The Journal of Neuroscience, July 2, 2014 • 34(27):9067–9075 • 9067 Development/Plasticity/Repair Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations Sarael Alcauter,1 Weili Lin,1 X J. Keith Smith,2 Sarah J. Short,3 Barbara D. Goldman,4 J. Steven Reznick,4 John H. Gilmore,3 and Wei Gao1 1Department of Radiology and Biomedical Research Imaging Center, 2Department of Radiology, 3Department of Psychiatry, and 4Frank Porter Graham Child Development Institute and Department of Psychology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 Although commonly viewed as a sensory information relay center, the thalamus has been increasingly recognized as an essential node in various higher-order cognitive circuits, and the underlying thalamocortical interaction mechanism has attracted increasing scientific interest. However, the development of thalamocortical connections and how such development relates to cognitive processes during the earliest stages of life remain largely unknown. Leveraging a large human pediatric sample (N ϭ 143) with longitudinal resting-state fMRI scans and cognitive data collected during the first 2 years of life, we aimed to characterize the age-dependent development of thalamo- cortical connectivity patterns by examining the functional relationship between the thalamus and nine cortical functional networks and determine the correlation between thalamocortical connectivity and cognitive performance at ages 1 and 2 years. Our results revealed that the thalamus–sensorimotor -

Magnetic Resonance Neurography of the Sciatic Nerve 197
Radiología. 2013;55(3):195---202 www.elsevier.es/rx UPDATE IN RADIOLOGY High resolution (3 T) magnetic resonance neurography of the ଝ sciatic nerve ∗ C. Cejas , M. Aguilar, L. Falcón, N. Caneo, M.C. Acuna˜ Servicio de Resonancia Magnética, Departamento de Diagnóstico por Imágenes, Fundación para la Lucha de las Enfermedades Neurológicas de la Infancia Dr. Raúl Carrea (FLENI), Buenos Aires, Argentina Received 4 November 2011; accepted 12 April 2012 KEYWORDS Abstract Magnetic resonance (MR) neurography refers to a set of techniques that enable Sciatic nerve; the structure of the peripheral nerves and nerve plexuses to be evaluated optimally. New Sciatic neuropathy; two-dimensional and three-dimensional neurographic sequences, in particular in 3 T scanners, Neurography; achieve excellent contrast between the nerve and perineural structures. MR neurography makes Magnetic resonance it possible to distinguish between the normal fascicular pattern of the nerve and anomalies like imaging inflammation, trauma, and tumor that can affect nerves. In this article, we describe the struc- ture of the sciatic nerve, its characteristics on MR neurography, and the most common diseases that affect it. © 2011 SERAM. Published by Elsevier España, S.L. All rights reserved. PALABRAS CLAVE Neurografía por resonancia magnética de alta resolución (3 Tesla) del nervio ciático Nervio ciático; Resumen La neurografía por resonancia magnética (RM) hace referencia a un conjunto de Neuropatía ciática; Neurografía; técnicas con capacidad para valorar óptimamente la estructura de los nervios periféricos y de los plexos nerviosos. Las nuevas secuencias neurográficas 2D y 3D, en particular en equipos Imagen por de 3 Tesla, consiguen un contraste excelente entre el nervio y las estructuras perineurales. -

Opposite Effects of Dopamine and Serotonin on Resting-State Networks: Review and Implications for Psychiatric Disorders
Molecular Psychiatry https://doi.org/10.1038/s41380-019-0406-4 REVIEW ARTICLE Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders 1,2 3 1,2,4,5 1,2 2,6,7 Benedetta Conio ● Matteo Martino ● Paola Magioncalda ● Andrea Escelsior ● Matilde Inglese ● 1,2 8,9,10 Mario Amore ● Georg Northoff Received: 15 September 2018 / Revised: 18 January 2019 / Accepted: 5 March 2019 © Springer Nature Limited 2019 Abstract Alterations in brain intrinsic activity—as organized in resting-state networks (RSNs) such as sensorimotor network (SMN), salience network (SN), and default-mode network (DMN)—and in neurotransmitters signaling—such as dopamine (DA) and serotonin (5-HT)—have been independently detected in psychiatric disorders like bipolar disorder and schizophrenia. Thus, the aim of this work was to investigate the relationship between such neurotransmitters and RSNs in healthy, by reviewing the relevant work on this topic and performing complementary analyses, in order to better understand their physiological link, as well as their alterations in psychiatric disorders. According to the reviewed data, neurotransmitters nuclei diffusively project to 1234567890();,: 1234567890();,: subcortical and cortical regions of RSNs. In particular, the dopaminergic substantia nigra (SNc)-related nigrostriatal pathway is structurally and functionally connected with core regions of the SMN, whereas the ventral tegmental area (VTA)-related mesocorticolimbic pathway with core regions of the SN. The serotonergic raphe nuclei (RNi) connections involve regions of the SMN and DMN. Coherently, changes in neurotransmitters activity impact the functional configuration and level of activity of RSNs, as measured by functional connectivity (FC) and amplitude of low-frequency fluctuations/temporal variability of BOLD signal.