Blood Glucose Testing CPT: 82947, 82948, 82962
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WELLNESS SCREENING TEST EXPLANATION SHEET We Encourage You to Share All Lab Results with Your Primary Care Provider
WELLNESS SCREENING TEST EXPLANATION SHEET We encourage you to share all lab results with your primary care provider. EXPLANATION OF BLOOD TESTS HEMOGLOBIN A1C: A Hemoglobin A1c level is HEMATOLOGY WELLNESS: expressed as a percent and is used to evaluate the Blood counts are often used as a broad screening test average amount of glucose in the blood over the last to determine an individual’s general health status. 2 to 3 months. This test may be used to screen for and White blood cells are the cells that are part of the diagnose diabetes or risk of developing diabetes. body’s defense system against infections and cancer and also play a role in allergies and inflammation. ALT: This test is used to determine if a patient has Red blood cells are the cells that transport oxygen liver damage. In healthy individuals, ALT levels in the throughout the body. Hemoglobin measures the blood are low and they rise in various disease states. total amount of the oxygen-carrying protein in the blood, which generally reflects the number of red CALCIUM: A calcium test is ordered to screen for, blood cells in the blood. Hematocrit measures the diagnose and monitor a range of conditions percentage of a person’s total blood volume that relating to the bones, heart, nerves, kidneys and consists of red blood cells. Platelets are cell fragments teeth. The test may also be ordered if a person has that are vital for normal blood clotting. symptoms of parathyroid disorder, malabsorption or an overactive thyroid. FREE T4: Free T4 is used to help evaluate thyroid function, generally after determining the TSH is CREATININE: The creatinine blood test is used to abnormal. -

Diabetes Control in Thyroid Disease
In Brief Thyroid disease is commonly found in most types of diabetes. This article defines the prevalence of thyroid disease in diabetes and elucidates through case studies the assessment, diagnosis, and clinical management of thyroid disease in diabetes. Diabetes Control in Thyroid Disease Thyroid disease is a pathological state abnormality. Several studies, including that can adversely affect diabetes con- the Colorado study, have documented trol and has the potential to negative- a higher prevalence of thyroid disease Jennal L. Johnson, MS, RNC, FNP, ly affect patient outcomes. Thyroid in women, with prevalence rates rang- BC-ADM, CDE disease is found commonly in most ing from 4 to 21%, whereas the rate in forms of diabetes and is associated men ranges from 2.8 to 16%.1 with advanced age, particularly in Thyroid disease increases with age. In type 2 diabetes and underlying the Colorado study, the 18-year-olds autoimmune disease in type 1 dia- had a prevalence rate of 3.5% com- betes. This article defines the preva- pared with a rate of 18.5% for those lence of thyroid disease in diabetes, ≥ 65 years of age. discusses normal physiology and The prevalence of thyroid disease in screening recommendations for thy- diabetes has been estimated at 10.8%,2 roid disease, and elucidates through with the majority of cases occurring as case studies the assessment, diagnosis, hypothyroidism (~ 30%) and subclini- and clinical management of thyroid cal hypothyroidism (~ 50%).2 Hyper- disease and its impact on diabetes. thyroidism accounts for 12%, and postpartum thyroiditis accounts for Thyroid Disease Prevalence 11%.2 Of the female patients with The prevalence of thyroid disease in type 1 diabetes, 30% have thyroid dis- the general population is estimated to ease, with a rate of postpartum thy- be 6.6%, with hypothyroidism the roid disease three times that of the most common malady.1 Participants normal population. -

Bilirubin As a New Biomarker of Diabetes and Its Microvascular
Analytica & l B y i tr o s c i h m e m e h i Biochemistry & s c t Nishimura, et al., Biochem Anal Biochem 2016, 5:1 o r i y B DOI: 10.4172/2161-1009.1000245 ISSN: 2161-1009 Analytical Biochemistry Commentary Open Access Bilirubin as a New Biomarker of Diabetes and its Microvascular Complications Takeshi Nishimura*, Masami Tanaka, Risa Sekioka and Hiroshi Itoh Department of Internal Medicine, School of Medicine, Keio University, Tokyo, Japan *Corresponding author: Takeshi Nishimura, Department of Internal Medicine, School of Medicine, Keio University 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan, Tel: 81-3-5363-3797; Fax: 81-3-3359-2745; E-mail: [email protected] Rec date: Feb 06, 2016; Acc date: Feb 12, 2016; Pub date: Feb 15, 2016 Copyright: © 2016 Nishimura T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Commentary elucidate the pathophysiological role of bilirubin in type 1 DM, our results should be verified in large-scale, prospective studies. Hyperglycemia is the hallmark of diabetes mellitus [DM]; it activates certain biochemical pathways leading to micro- and macrovascular complications in diabetic patients [1]. Moreover, References hyperglycemia generates oxidative stress and causes free radical- 1. Takayanagi R, Inoguchi T, Ohnaka K (2011) Clinical and experimental mediated lipid peroxidation [2,3]. In turn, the oxidative stress causes evidence for oxidative stress as an exacerbating factor of diabetes mellitus. -

Diagnosis of Insulinoma in a Maine Coon
JOR0010.1177/2055116919894782Journal of Feline Medicine and Surgery Open ReportsGifford et al 894782research-article2020 Case Report Journal of Feline Medicine and Surgery Open Reports Diagnosis of insulinoma in 1 –10 © The Author(s) 2020 Article reuse guidelines: a Maine Coon cat sagepub.com/journals-permissions DOI:https://doi.org/10.1177/2055116919894782 10.1177/2055116919894782 journals.sagepub.com/home/jfmsopenreports This paper was handled and processed by the American Editorial Office (AAFP) 1 2 3 Carol H Gifford , Anita P Morris , Kurt J Kenney and for publication in JFMS Open Reports J Scot Estep4 Abstract Case summary A 9-year-old male neutered Maine Coon cat presented with a 6-month history of polyphagia and one recent episode of tremors and weakness. Blood work revealed profound hypoglycemia and results of a paired insulin glucose test were consistent with an insulinoma. Abdominal ultrasound revealed a solitary pancreatic mass, and results of a fine-needle aspirate (FNA) gave further support for the location of the neuroendocrine tumor. After unsuccessful medical management of the hypoglycemia, the mass was surgically removed. Immunohistochemistry confirmed that it was an insulinoma. At the time of writing, the patient had been in clinical remission for 9 months. Relevance and novel information Feline insulinomas are rare and there is very little information on their behavior, clinical course and histologic characteristics. This is the first reported case of an insulinoma in a Maine Coon cat and the first to describe results of an ultrasound-guided FNA of the mass. In addition, the progression of disease, histopathology and immunohistochemistry results add to the currently minimal database for feline insulinomas. -

Levels of Serum Uric Acid in Patients with Impaired Glucose Tolerance
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 08, ISSUE 02, 2021 LEVELS OF SERUM URIC ACID IN PATIENTS WITH IMPAIRED GLUCOSE TOLERANCE 1,2Shahzad Ali Jiskani*, 1Lubna Naz, 3Ali Akbar Pirzado, 4Dolat Singh, 5Asadullah Hanif, 6Razia Bano 1Department of Pathology, Chandka Medical College, SMBBMU, Larkano 2Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan 3Department of Community Medicine and Public Health, Chadka Medical College, SMBBMU, Larkana 4Department of Medicine, Indus Medical College Hospital, Tando Muhammad Khan 5Health Department, Govt. of Sindh 6Department of Medicine, Liaquat University of Medical & Health Sciences Jamshoro *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Chandka Medical College, SMBBMU, Larkano ABSTRACT Objective: The main objective of this study is to see levels of serum uric acid in patients having impaired glucose tolerance as compared to normal glucose tolerant individuals. Patients and Methods: This was prospective study, conducted at Department of Medicine and Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan for the period of 8 months (March 2019 to October 2019). A total of 140 patients were included in this study. An oral glucose test was performed with load of 75g glucose in patients having impaired glucose in fasting. Both normal glucose tolerance and impaired glucose tolerance individuals were evaluated for their serum uric acid level. Serum glucose levels and serum uric acid levels were performed on COBAS C-111 Chemistry Analyzer ensuring procedures of quality control. Data was entered and analyzed using SPSS 21.0. Results: In patients having impaired glucose tolerance, the frequency of hyperuricemia was 62.35%. Analysis of both groups (patients with normal glucose tolerance and glucose intolerance), association was strong with p-value of <0.001. -

SIRS Is Valid in Discriminating Between Severe and Moderate Diabetic Foot Infections
Pathophysiology/Complications ORIGINAL ARTICLE SIRS Is Valid in Discriminating Between Severe and Moderate Diabetic Foot Infections 1 2 DANE K. WUKICH, MD KATHERINE MARIE RASPOVIC, DPM best of our knowledge, the use of SIRS 2 3 KIMBERLEE B. HOBIZAL, DPM BEDDA L. ROSARIO, PHD has not yet been validated as a method of discriminating between moderate and severe DFI. OBJECTIVEdThis retrospective, single-center study was designed to distinguish severe di- The aim of this study was to classify abetic foot infection (DFI) from moderate DFI based on the presence or absence of systemic fl infectionseverityinagroupofhospital- in ammatory response syndrome (SIRS). ized diabetic patients based on the pres- RESEARCH DESIGN AND METHODSdThe database of a single academic foot and ence or absence of SIRS. The reason for ankle program was reviewed and 119 patients were identified. Severe DFI was defined as local hospitalization in this group of patients infection associated with manifestation of two or more objective findings of systemic toxicity was their DFI. Our hypotheses are that using SIRS criteria. patients with DFI who manifest SIRS (i.e., severe infection) will have longer hospital RESULTSdPatients with severe DFI experienced a 2.55-fold higher risk of any amputation – – stays and higher rates of major amputa- (95% CI 1.21 5.36) and a 7.12-fold higher risk of major amputation (1.83 41.05) than patients tion than patients who don’tmanifest with moderate DFI. The risk of minor amputations was not significantly different between the two groups (odds ratio 1.02 [95% CI 0.51–2.28]). The odds of having a severe DFI was 7.82 SIRS (i.e., moderate infection). -

C-Reactive Protein and Gamma-Glutamyltransferase
Hindawi Publishing Corporation Experimental Diabetes Research Volume 2010, Article ID 761715, 8 pages doi:10.1155/2010/761715 Clinical Study C-Reactive Protein and Gamma-Glutamyltransferase Concentrations in Relation to the Prevalence of Type 2 Diabetes Diagnosed by Glucose or HbA1c Criteria in Chinese Adults in Qingdao, China J. Ren,1 Z. C. Pang,2 W. G. Gao,3, 4, 5 H. R. Nan,6 S. J. Wang,2 L. Zhang,3, 4, 5 and Q. Qiao3, 4 1 Department of Epidemiology and Health Statistics, Shandong University, Jinan 250012, China 2 Department of Non-Communicable Disease Prevention, Qingdao Municipal Centre for Disease Control and Prevention, no. 175, Shandong Road, Qingdao 266071, China 3 Department of Public Health, University of Helsinki, 00014 Helsinki, Finland 4 Diabetes Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, 00014 Helsinki, Finland 5 Qingdao Endocrinology and Diabetes Hospital, 266071 Qingdao, China 6 Hong Kong Institute of Diabetes and Obesity, Hong Kong, China CorrespondenceshouldbeaddressedtoZ.C.Pang,[email protected] Received 13 August 2010; Revised 12 October 2010; Accepted 12 October 2010 Academic Editor: Giuseppe Paolisso Copyright © 2010 J. Ren et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aims. To investigate the association of C-reactive protein (CRP) and gamma glutamyltransferase (GGT) concentrations with newly diagnosed diabetes defined by either glucose or HbA1c criteria in Chinese adults. Methods. A population-based cross-sectional study was conducted in 2006. Data from 1167 men and 1607 women aged 35–74 years were analyzed. -
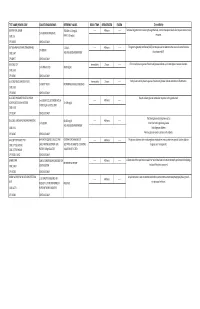
G's- Lab Test Menu
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility GENTAMICIN, SERUM TROUGH: 0-2.0 mg/dL ------ 48 hours ------ Tests level of gentamicin circulating through the body. Certain therapeutic levels elicit require anti-microbial 2 ml SERUM. REFRIGERATE. CODE: GE PEAK: 5-10 mg/dL response. CPT: 81070 SCHEDULE: DAILY GGT (GAMMA GLUTAMYL TRANSFERASE) 5-31 IU/L ------ 48 hours ------ The gamma-glutamyl transferase (GGT) test may be used to determine the cause of elevated alkaline 1ml SERUM CODE: GGT AGE AND GENDER DEPENDENT phosphatase (ALP). CPT:82977 SCHEDULE: DAILY GLUCOSE, CSF Immediately 2 hours ------ CFS normally has no glucose. Presence of glucose indicates spinal meningitis or seizure disorders. 1 ml SPINAL FLUID 40-80 mg/mL CODE: GLFL CPT: 82947 SCHEDULE: DAILY GLUCOSE, MISCELLANEOUS FLUID Immediately 2 hours ------ Body fluids normally have no glucose. Presence of glucose indicates infection or inflammation. 1 ml BODY FLUID NO NORMAL RANGE ESTABLISHED CODE: GLFL CPT: 82945 SCHEDULE: DAILY GLUCOSE, PREGNANT RELATED 1 HOUR Results indicate glucose metabolism response to the glucola load. 1 ml SERUM COLLECTED ONE HOUR ------ 48 hours ------ LOADING (O'SULLIVAN SCREEN) 70-139 mg/dL AFTER 50 gm GLUCOSE LOAD CODE: GESC CPT: 82947 SCHEDULE: DAILY The blood glucose test may be used to: GLUCOSE, SERUM (FASTING AND RANDOM) 60-115 mg/dL ------ 48 hours ------ 1 ml SERUM Screen for both high blood glucose AGE AND GENDER DEPENDENT CODE: GLU Help diagnose diabetes CPT: 82947 SCHEDULE: DAILY Monitor glucose levels in persons with diabetes GLUCOSE TOLERANCE TEST 8HR FAST REQUIRED. COLLECT AND CRITERIA FOR DIAGNOSIS OF ------ 48 hours ------ The glucose tolerance test monitors glucose metabolism over a certain time period. -

Correlation Between Glycated Hemoglobin and Venous Blood Sugar in Diabetic Patients Monitored in Abidjan
Vol. 14(4), pp. 135-141, October-December 2020 DOI: 10.5897/AJBR2020.1102 Article Number: CD65C6E65033 ISSN 1996-0778 Copyright © 2020 Author(s) retain the copyright of this article African Journal of Biochemistry Research http://www.academicjournals.org/AJBR Full Length Research Paper Correlation between glycated hemoglobin and venous blood sugar in diabetic patients monitored in Abidjan MONDE Aké Absalome1*, CAMARA-CISSE Massara2, KOFFI Konan Gervais2, DIALLO Issiagha3, AKE AKE Alexandre4, YAPO-AKE Bénédicte4, ECRABEY Yann Christian3, KOUAKOU Francisk3, BENE YAO Roger Espérance4 and TIAHOU Georges5 1Félix HOUPHOUËT-BOIGNY University, Cocody, Abidjan, Côte d’Ivoire. 2Biochemistry Laboratory, Abidjan Medical School, Félix HOUPHOUËT BOIGNY University, Côte D'ivoire. 3Biochemistry Laboratory, University Hospital Center of Treichville, Côte D'ivoire. 4Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Félix HOUPHOUËT-BOIGNY University, Côte D'ivoire. 5Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Alassane OUATTARA University, Bouaké, Côte D'ivoire. Received 23 August, 2020; Accepted 2 October, 2020 The aim of this study was to determine the correlation between glycated hemoglobin and blood sugar levels in diabetic subjects carried out in Abidjan. This cross-sectional study included 100 patients with diabetes monitored, for three months, for whom glycated blood glucose and hemoglobin were performed, this after informed consent of the patients. Pearson and Spearman correlation tests were used, at the 5% threshold. The patients with normal HbA1C and normal blood glucose accounted for 55.34 and 32%, respectively. A sedentary lifestyle and body mass index > 25 kg/m² were associated with a significant increase in the risk of increased blood glucose and HbA1C. -

Glycation of Fetal Hemoglobin Reflects Hyperglycemia Exposure
2830 Diabetes Care Volume 37, October 2014 Felix O. Dupont,1 Marie-France Hivert,1,2,3 Glycation of Fetal Hemoglobin Catherine Allard,1 Julie Menard,´ 1 Patrice Perron,1,2 Luigi Bouchard,1,4,5 Reflects Hyperglycemia Exposure Julie Robitaille,6 Jean-Charles Pasquier,1,7 Christiane Auray-Blais,1,8 and In Utero Jean-Luc Ardilouze1,2 Diabetes Care 2014;37:2830–2833 | DOI: 10.2337/dc14-0549 OBJECTIVE The lifetime risk of metabolic diseases in offspring of women with gestational diabetes mellitus (GDM) depends, at least in part, on the impact of glycemic fetal programming. To quantify this impact, we have developed and validated a unique mass spectrometry method to measure the percentage of glycated hemoglobin in cord blood. RESEARCH DESIGN AND METHODS This case-control study includes 37 GDM women and 30 pregnant women with normal glucose tolerance (NGT). RESULTS Glycation of the a-chain (Gla) was higher in neonates from GDM (2.32 vs. 2.20%, P < 0.01). Gla strongly correlated with maternal A1C measured at delivery in the r P < r P < overall cohort ( =0.67, 0.0001) as well as in each group (GDM: = 0.66, 1 r P Centre de Recherche du Centre Hospitalier Uni- 0.0001; NGT: = 0.50, = 0.01). versitaire de Sherbrooke, Sherbrooke, QC, Canada 2Endocrine Division, Universite´ de Sherbrooke, CONCLUSIONS Sherbrooke, QC, Canada Thus, Gla may reflect hyperglycemic exposure during the last weeks of fetal de- 3Harvard Pilgrim Health Care Institute, Depart- velopment. Future studies will confirm Gla is a predictive biomarker of prenatally ment of Population Medicine, Harvard Medical School, Boston, MA programmed lifetime metabolic health and disease. -

Glucose Metabolism Before and After Radioiodine Therapy of a Patient with Graves' Disease: Assessment by Continuous Glucose Monitoring
BIOMEDICAL REPORTS 7: 183-187, 2017 Glucose metabolism before and after radioiodine therapy of a patient with Graves' disease: Assessment by continuous glucose monitoring YUN HU, GU GAO, RENG-NA YAN, FENG-FEI LI, XIAO-FEI SU and JIAN-HUA MA Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu 210012, P.R. China Received March 17, 2017; Accepted June 7, 2017 DOI: 10.3892/br.2017.932 Abstract. Hyperthyroidism causes impaired glucose toler- as well as a diagnostic test for diabetes mellitus. However, ance, insulin resistance (IR) and insulin secretion. However, previous studies identified that HbA1c is not a reliable diag- the glucose variability affected by thyroid dysfunction remains nostic test for diabetes in the presence of hyperthyroidism or unclear. Glucose variability was assessed by continuous hypothyroidism (9,10). glucose monitoring (CGM) in a non-diabetic patient with The prevalent use of continuous glucose monitoring Graves' disease (GD), to the best of our knowledge, for the (CGM) has provided details on glycemic variation that were first time. A 28‑year‑old man with GD, who had been taking otherwise not possible with HbA1c or self-monitoring of methimazole for 4 years, was treated with radioiodine on blood glucose (SMBG). Torimoto et al (11) reported the use August 17th 2016. Although the patient exhibited normal of CGM in a patient with Graves' disease (GD) complicated glycated hemoglobin (HbA1c; 5.3%) and blood glucose values by diabetes mellitus. The authors demonstrated that hyper- during the oral glucose tolerance test (OGTT; fasting and thyroidism induces elevation of postprandial blood glucose 120 min blood glucose were 5.38 and 6.39 mmol/l, respec- (PBG) and fasting blood glucose (FBG) levels due to the dawn tively) before radioiodine therapy, CGM exhibited high 24 h phenomenon, and that glycemic variation is alleviated with mean glucose and nocturnal hyperglycemia. -

Clinical Performance of Flash Glucose Monitoring System in Patients With
www.nature.com/scientificreports OPEN Clinical Performance of Flash Glucose Monitoring System in Patients with Liver Cirrhosis and Diabetes Mellitus Dalila Costa1,2 ✉ , Joana Lourenço1, Ana Margarida Monteiro3, Beatriz Castro1, Patricia Oliveira3, Maria Carmo Tinoco3, Vera Fernandes3, Olinda Marques3, Raquel Gonçalves2 & Carla Rolanda1,2 Flash glucose monitoring system (FGMS) is an improved subset of continuous glucose monitoring with a recognized efectiveness on glycemic control, though validation in patients with Liver Cirrhosis (LC) is lacking. To evaluate the accuracy of FGMS in patients with Type 2 Diabetes Mellitus (DM) and LC, a prospective, case-control study was performed in 61 ambulatory patients with LC and DM (LC group, n = 31) or DM (Control group, n = 30). During 14 days, patients performed 4 assessments per day of self-monitoring of blood glucose (SMBG, reference value) followed by FGMS scanning. There were 2567 paired SMBG and FGMS values used in the accuracy analysis, with an overall mean absolute relative diference (MARD) of 12.68% in the LC group and 10.55% in the control group (p < 0,001). In patients with LC, the percentage of readings within Consensus Consensus Error Grid analysis Zone A and A + B were 80.36% and 99,26%, respectively. Sensor clinical accuracy was not afected by factors such as body mass index, age, gender, Child-Pugh score or edematoascitic decompensation. This is the frst study to approach FGMS clinical accuracy in LC, revealing a potential usability of this system to monitor glycemic control in this population. Type 2 Diabetes Mellitus (DM) has been considered a Public Health challenge due to its signifcant impact on mortality and morbidity worldwide1.