Laser Mass Spectrometric Detection of Extraterrestrial SPECIAL FEATURE Aromatic Molecules: Mini-Review and Examination of Pulsed Heating Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Messengers from Outer Space
The complete absence of national laboratories for standardizing radiation measurements and the lack of physics departments in most radiotherapy centres in Latin America warrant the setting up of one or more regional dosimetry facilities, the functions of which would be primarily to calibrate dosimeters; to provide local technical assistance by means of trained staff; to check radiation equipment and dosimeters; to arrange a dose-intercomparison service; and to co-operate with local personnel dosimetry services. To be most effective, this activity should be in the charge of local personnel, with some initial assistance in the form of equipment and experts provided by the Agency. Although the recommendations were presented to the IAEA as the or ganization responsible for the setting up of the panel, the participants re commended that the co-operation of the World Health Organization and the Pan-American Health Organization should be invited. It was also suggested that the panel report be circulated to public health authorities in the countries represented. MESSENGERS FROM OUTER SPACE Although no evidence has yet been confirmed of living beings reaching the earth from space, it has been estimated that hundreds of tons of solid matter arrive every day in the form of meteorites or cosmic dust particles. Much is destroyed by heat in the atmosphere but fragments recovered can give valuable information about what has been happening in the universe for billions of years. Some of the results of worldwide research on meteorites were given at a symposium in Vienna during August. During six days of discussions a total of 73 scientific papers was present ed and a special meeting was held for the purpose of improving international co-operation in the research. -
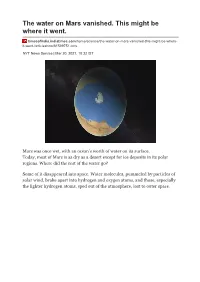
The Water on Mars Vanished. This Might Be Where It Went
The water on Mars vanished. This might be where it went. timesofindia.indiatimes.com/home/science/the-water-on-mars-vanished-this-might-be-where- it-went-/articleshow/81599751.cms NYT News Service | Mar 20, 2021, 10:32 IST Mars was once wet, with an ocean’s worth of water on its surface. Today, most of Mars is as dry as a desert except for ice deposits in its polar regions. Where did the rest of the water go? Some of it disappeared into space. Water molecules, pummeled by particles of solar wind, broke apart into hydrogen and oxygen atoms, and those, especially the lighter hydrogen atoms, sped out of the atmosphere, lost to outer space. A tall outcropping of rock, with layered deposits of sediments in the distance, marking a remnant of an ancient, long-vanished river delta in Jezero Crater, are pictured in this undated image taken by NASA's Mars rover Perseverance. (Reuters) But most of the water, a new study concludes, went down, sucked into the red planet’s rocks. And there it remains, trapped within minerals and salts. Indeed, as much as 99% of the water that once flowed on Mars could still be there, the researchers estimated in a paper published this week in the journal Science. Data from the past two decades of robotic missions to Mars, including NASA ’s Curiosity rover and the Mars Reconnaissance Orbiter, showed a wide distribution of what geologists call hydrated minerals. “It became very, very clear that it was common and not rare to find evidence of water alteration,” said Bethany Ehlmann, a professor of planetary science at the California Institute of Technology and one of the authors of the paper. -

The Protection of Frequencies for Radio Astronomy 1
JOURNAL OF RESEARCH of the National Bureau of Standards-D. Radio Propagation Vol. 67D, No. 2, March- April 1963 b The Protection of Frequencies for Radio Astronomy 1 R. 1. Smith-Rose President, International Scientific Radio Union (R eceived November 5, 1962) The International T elecommunications Union in its Geneva, 1959 R adio R egulations r recognises the Radio Astronomy Service in t he two following definitions: N o. 74 Radio A st1" onomy: Astronomy based on t he reception of waves of cos mi c origin. No. 75 R adio A st1"onomy Se1"vice: A service involving the use of radio astronomy. This service differs, however, from other r adio services in two important respects. 1. It does not itself originate any radio waves, and therefore causes no interference to any other service. L 2. A large proportion of its activity is conducted by the use of reception techniques which are several orders of magnit ude )]).ore sensitive than those used in other ra dio services. In order to pursue his scientific r esearch successfully, t he radio astronomer seeks pro tection from interference first, in a number of bands of frequencies distributed t hroughout I t he s p ~ct run:; and secondly:. 1~10r e complete and s p ec i~c prote.ction fOl: t he exact frequency bands III whIch natural radIatIOn from, or absorptIOn lD, cosmIc gases IS known or expected to occur. The International R egulations referred to above give an exclusive all ocation to one freq uency band only- the emission line of h ydrogen at 1400 to 1427 Mc/s. -

What Are Cosmic Rays?!
WhatWWhatWhhaatt areaarearree CosmicCCosmicCoossmmiicc Rays?!RRays?!Raayyss??!! By Hayanon Translated by Y. Noda and Y. Kamide Supervised by Y. Muraki ᵶᵶᵋᵰᵿᶗᶑᵘᴾᴾᵱᶇᶀᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑᵋᵰᵿᶗᶑᵘᵘᴾᴾᵱᶇᶀᶊᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑ Have you ever had an X-ray examination Scientists identified three types at the hospital? In 1896, a German of radiation: positively-charged alpha physicist, W. C. Röntgen, astonished people particles, negatively-charged beta particles, with an image of bones captured through and uncharged gamma rays. In 1903, M. the use of X-rays. He had just discovered Curie along with her husband, P. Curie, and the new type of rays emitted by a discharge Becquerel, won the Nobel prize in physics. device. He named them X-rays. Because Furthermore, M. Curie was awarded the of their high penetration ability, they are Nobel prize in chemistry in 1911. able to pass through flesh. Soon after, it Certain types of radiation including was found that excessive use of X-rays can X-rays are now used for many medical cause harm to bodies. purposes including examining inside the In that same year, a French scientist, body, treating cancer, and more. Radiation, A. H. Becquerel, found that a uranium however, could be harmful unless the compound also gave off mysterious rays. amount of radiation exposure is strictly To his surprise, they could penetrate controlled. wrapping paper and expose a photographic The work with radium by M. Curie later film generating an image of the uranium led to the breakthrough discovery of compound. The uranium rays had similar the radiation coming from space. These characteristics as those of X-rays, but were cosmic rays were discovered by an Austrian determined to be different from them. -

NASA Astrobiology Institute 2018 Annual Science Report
A National Aeronautics and Space Administration 2018 Annual Science Report Table of Contents 2018 at the NAI 1 NAI 2018 Teams 2 2018 Team Reports The Evolution of Prebiotic Chemical Complexity and the Organic Inventory 6 of Protoplanetary Disk and Primordial Planets Lead Institution: NASA Ames Research Center Reliving the Past: Experimental Evolution of Major Transitions 18 Lead Institution: Georgia Institute of Technology Origin and Evolution of Organics and Water in Planetary Systems 34 Lead Institution: NASA Goddard Space Flight Center Icy Worlds: Astrobiology at the Water-Rock Interface and Beyond 46 Lead Institution: NASA Jet Propulsion Laboratory Habitability of Hydrocarbon Worlds: Titan and Beyond 60 Lead Institution: NASA Jet Propulsion Laboratory The Origins of Molecules in Diverse Space and Planetary Environments 72 and Their Intramolecular Isotope Signatures Lead Institution: Pennsylvania State University ENIGMA: Evolution of Nanomachines in Geospheres and Microbial Ancestors 80 Lead Institution: Rutgers University Changing Planetary Environments and the Fingerprints of Life 88 Lead Institution: SETI Institute Alternative Earths 100 Lead Institution: University of California, Riverside Rock Powered Life 120 Lead Institution: University of Colorado Boulder NASA Astrobiology Institute iii Annual Report 2018 2018 at the NAI In 2018, the NASA Astrobiology Program announced a plan to transition to a new structure of Research Coordination Networks, RCNs, and simultaneously planned the termination of the NASA Astrobiology Institute -

Problems with Panspermia Or Extraterrestrial Origin of Life Scenarios As Found on the IDEA Center Website At
Problems with Panspermia or Extraterrestrial Origin of Life Scenarios As found on the IDEA Center website at http://www.ideacenter.org Panspermia is the theory that microorganisms or biochemical compounds from outer space are responsible for originating life on Earth and possibly in other parts of the universe where suitable atmospheric conditions exist. Essentially, it is a hypothesis which states that life on earth came from outer space. It has been popularized in countless science fiction works, however some scientists, including the discoverer of the structure of DNA, Francis Crick, have advocated panspermia. There are generally about 3 different "flavors" of panspermia: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. 2. Directed Panspermia where intelligent life on other planets intentionally seeded other planets with their own form of life. 3. Intelligent Design Panspermia, where intelligent beings from another planet came to earth and designed life here. Each type of panspermia will be briefly discussed below: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. Naturalistic panspermia has gained popularity because some have recognized that life on earth appears very soon after the origin of conditions which would allow it to exist. Many scientists believed that if life had originated on earth it would have taken many hundreds of millions, or even billions of years to do so. Life appears perhaps less than 200 million after the origin of conditions at which life could exist. -

Owning Outer Space Ezra J
Northwestern Journal of International Law & Business Volume 20 Issue 1 Fall Fall 1999 Owning Outer Space Ezra J. Reinstein Follow this and additional works at: http://scholarlycommons.law.northwestern.edu/njilb Part of the Air and Space Law Commons Recommended Citation Ezra J. Reinstein, Owning Outer Space, 20 Nw. J. Int'l L. & Bus. 59 (1999-2000) This Article is brought to you for free and open access by Northwestern University School of Law Scholarly Commons. It has been accepted for inclusion in Northwestern Journal of International Law & Business by an authorized administrator of Northwestern University School of Law Scholarly Commons. Owning Outer Space Ezra J. Reinstein * I. INTRODUCTION What do we want from space? We want the knowledge we can gain from scientific research; we can learn much about the Earth and its inhabitants, as well as the universe around us, by studying space. We want to explore, to satisfy the thirst for adventure and conquest imagined in countless science fiction books and films. We want to improve our collective lot down here on Earth. Space offers the potential for practically limitless wealth, some already being exploited, some we may only harness in the distant future, and un- doubtedly some we cannot begin to guess. Already the wealth of space is being developed in the form of telecommunications and remote satellite ob- servation. The private-sector investment in telecommunications satellites alone was projected to total $54.3 billion (including launch) between 1996 and 20001 -- and this figure doesn't include other commercial space ven- tures, nor does it include investment in Russian and Chinese satellites. -

Phd Projects at the Institute of Origins
PhD projects at the Institute of Origins. A list of possible PhD projects at the Institute of Origins appear in the following pages. If you have any questions regarding any projects please contact the individual supervisors. Also if you have other suggestions for a project please contact us as well. The chemical composition of star forming regions near and far .................................... 3 ! Modelling the solubilities of organic solids in hydrocarbon liquids: application to the geology and astrobiology of Titan. .................................................................................... 4! Modeling turbulent flows in solar quiescent prominences ...............................................5! The zoo of exo-planets..................................................................................................8! Understanding the formation of heavy negative ions at Titan and Enceladus................9! Mapping anthropogenic versus natural sources of atmospheric CO2 ............................11! Probing Large Scale Structure with High Energy Neutrinos.........................................13! Future Moon Missions and High Energy Neutrinos ......................................................15! Measuring Cosmic Particles and the Upper Atmosphere with LOFAR.........................17! Mimicking planetary environments for assessing the survivability of bacterial organisms within an artificial environmental chamber. A combined planetary atmosphere and microbiological study for exploring panspermia................................ -

Crater: How Cosmic Rays Affect Humans
Lunar Reconnaissance Orbiter: (CRaTER) Audience CRaTER: Grades 6-8 How Cosmic Rays Affect Time Recommended 30-45 minutes Humans AAAS STANDARDS • 1B/1: Scientific investigations usually involve the col- lection of relevant evidence, the use of logical reasoning, Learning Objectives: and the application of imagination in devising hypoth- • Students will be able to describe why cosmic rays are dangerous to eses and explanations to make sense of the collected evidence. astronauts. • Students will learn to design a scientific instrument. • 3A/M2: Technology is essential to science for such purposes as access to outer space and other remote • Students will think critically about how to protect astronauts from cosmic locations, sample collection and treatment, measurement, rays. data collection and storage, computation, and communi- cation of information. NSES STANDARDS Preparation: Content Standard A (5-8): Abilities necessary to do scien- None tific inquiry: c. Use appropriate tools to gather, analyze and inter- pret data. Background Information: d. Develop descriptions and explanations using On their trips to and from the Moon, Apollo astronauts saw small white flashes evidence. of light while in the dark—even with their eyes closed. They usually saw no e. Think critically and logically to make relationships more than a couple each minute, although at least one astronaut saw so many between evidence and explanations he had trouble sleeping. What caused these flashes? Content Standard E (5-8): Science and Technology: b. Technology is essential to science, because it The answer is cosmic rays. Because of their high speeds, thousands of these provides instruments and techniques that enable observations of objects and phenomena that cosmic rays were passing through the bodies of the Apollo astronauts every are otherwise unobservable due to factors such second. -

Ames Research Center
Research and Technology NASA Ames Research Research and Technology Ames Research Center National Aeronautics and Space Administration Ames Research Center Moffett Field, California NASA TM-112195 Foreword The mission of NASA Ames Research Center is to research, develop, verify, and transfer advanced aeronautics, space, and related technologies; to advance and communicate scientific knowledge and understanding of the universe, the solar system, and the Earth; and to enable the development of space for human enterprise. Empha- sis is placed on information systems technologies for aeronautics and space applications; on aviation operations systems; and on the discipline of astrobiology, the study of life in the universe encompassing the Earth, space, and life sciences. This report highlights the challenging work accomplished during fiscal year 1996 by Ames research scientists, engineers, and technologists. It discusses research and technologies that enable the Information Age, that expand the frontiers of knowledge for aeronautics and space, and that help to maintain U. S. leadership in aeronautics and space research and technology development. The accomplishments span the range of goals of NASA’s four Strategic Enterprises: Aeronautics and Space Transportation Technology, Space Science, Human Exploration and Development of Space, and Mission to Planet Earth. The primary purpose of this report is to communicate information—to inform our stakeholders, customers, and partners, and the people of the United States about the scope and diversity of Ames’ mission, the nature of Ames’ research and technology activities, and the stimulating challenges ahead. The accomplishments cited illustrate the contributions that Ames is making to improve the quality of life for our citizens and the economic position of the United States in the world marketplace. -

Carl Sagan 1934–1996
Carl Sagan 1934–1996 A Biographical Memoir by David Morrison ©2014 National Academy of Sciences. Any opinions expressed in this memoir are those of the author and do not necessarily reflect the views of the National Academy of Sciences. CARL SAGAN November 9, 1934–December 20, 1996 Awarded 1994 NAS Pubic Welfare Medal Carl Edward Sagan was a founder of the modern disci- plines of planetary science and exobiology (which studies the potential habitability of extraterrestrial environments for living things), and he was a brilliant educator who was able to inspire public interest in science. A visionary and a committed defender of rational scientific thinking, he transcended the usual categories of academia to become one of the world’s best-known scientists and a true celebrity. NASA Photo Courtesy of Sagan was propelled in his careers by a wealth of talent, By David Morrison a large share of good luck, and an intensely focused drive to succeed. His lifelong quests were to understand our plane- tary system, to search for life beyond Earth, and to communicate the thrill of scientific discovery to others. As an advisor to the National Aeronautics and Space Administration (NASA) and a member of the science teams for the Mariner, Viking, Voyager, and Galileo missions, he was a major player in the scientific exploration of the solar system. He was also a highly popular teacher, but his influence reached far beyond the classroom through his vivid popular writing and his mastery of the medium of television. The early years Born in 1934, Sagan grew up in a workingclass Jewish neighborhood of Brooklyn, New York, and attended public schools there and in Rahway, New Jersey. -

Constraining Prebiotic Chemistry Through a Better Understanding of Earth’S Earliest Environments
Constraining prebiotic chemistry through a better understanding of Earth’s earliest environments Timothy W. Lyons (University of California, Riverside), Phone: 951-827-3106, Email: [email protected] Co-authors: Karyn Rogers (Rensselaer Polytechnic Institute), Ramanarayanan Krishnamurthy (Scripps Research Institute), Loren Williams (Georgia Institute of Technology), Simone Marchi (Southwest Research Institute), Edward Schwieterman (Univ. of California, Riverside), Dustin Trail (Univ. of Rochester), Noah Planavsky (Yale Univ.), Christopher Reinhard (Georgia Institute of Technology) Co-signatories: Vladimir Airapetian (NASA GSFC/American Univ.), Jan Amend (Univ. of Southern California), Ariel Anbar (Arizona State Univ.), Jose Aponte (NASA GSFC/Catholic Univ. of America), Giada Arney (NASA GSFC), Laura Barge (NASA Jet Propulsion Laboratory, California Institute of Technology), Steven Benner (Foundation for Applied Molecular Evolution), Roy Black (Univ. of Washington), Dennis Bong (Ohio State Univ.), Donald Burke-Aguero (Univ. of Missouri), Jeffrey Catalano (Washington Univ.), David Catling (Univ. of Washington), George Cody (Carnegie Institution for Science), Jason Dworkin (NASA GSFC), Jamie Elsila (NASA GSFC), Paul Falkowski (Rutgers Univ.), Daniel Glavin (NASA GSFC), Heather Graham (NASA GSFC/Catholic Univ. of America), Nicholas Hud (Georgia Institute of Technology), James Kasting (Penn State Univ.), Sarah Keller (Univ. of Washington), Jun Korenaga (Yale Univ.), Ravi Kumar Kopparapu (NASA GSFC), Susan Lang (Univ. of South Carolina),