Histochemical Assays of Secretory Trichomes and the Structure and Content of Selected Components in Rubus Idaeus L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rasprostranjenost Roda Rubus U Hrvatskoj
Dubravka Dujmović Purgar i sur.; Rasprostranjenost roda Rubus u Hrvatskoj Rasprostranjenost roda Rubus u Hrvatskoj Distribution of genus Rubus in Croatia Dubravka Dujmović Purgar, Zoran Šindrak, Sandra Voća, Ivica Šnajder, Aleš Vokurka, Boris Duralija SAŽETAK Zahvaljujući klimatskim uvjetima i zemljopisnom položaju, Hrvatska flora je bogata biljnim vrstama. Među njima postoji velik broj samoniklih voćnih vrsta, zanimljivih zbog natprosječne kakvoće svojih plodova. Podaci iz postojeće literature kao i oni vlastitih istraživanja uspoređeni su s popisom svojti rasprostranjenih u Europi. Zbog jednostavnog međuvrsnog križanja svojti roda Rubus, postoji velika genetska raznolikost unutar roda koja je u prošlosti korištena za izravni odabir iz prirodnih populacija i stvaranje brojnih sorti. Neke od tih vrsta mogu se rabiti i u budućim oplemenjivačkim programima. Ključne riječi: Rubus, flora, prirodne populacije, genetska raznolikost, Hrvatska ABSTRACT Thanks to its climatic conditions and geographic location, Croatian flora is rich in plant species. Among existing plants there is a distinct number of autochthonous edible fruit species interesting for their outstanding fruit quality. Data from existing Croatian literature and data from our own investigations were compared with the list of Rubus spp. plant taxa that grow in Europe. Easy interspecific hybridization of Rubus species is responsible for high genetic variety of Rubus spp., which was in the past used for direct selection in natural populations and breeding of various cultivars all over the world. It could also be used in future fruit breeding programmes, practically without limitations. Key words: Rubus, flora, natural populations, genetic variety, Croatia UVOD Vaskularna flora Hrvatske je među bogatijima u Europi s 5593 vrste koje su rasprostranjene u svim njenim regijama (Nikolić 2006). -

Plant List for VC54, North Lincolnshire
Plant List for Vice-county 54, North Lincolnshire 3 Vc61 SE TA 2 Vc63 1 SE TA SK NORTH LINCOLNSHIRE TF 9 8 Vc54 Vc56 7 6 5 Vc53 4 3 SK TF 6 7 8 9 1 2 3 4 5 6 Paul Kirby, 31/01/2017 Plant list for Vice-county 54, North Lincolnshire CONTENTS Introduction Page 1 - 50 Main Table 51 - 64 Summary Tables Red Listed taxa recorded between 2000 & 2017 51 Table 2 Threatened: Critically Endangered & Endangered 52 Table 3 Threatened: Vulnerable 53 Table 4 Near Threatened Nationally Rare & Scarce taxa recorded between 2000 & 2017 54 Table 5 Rare 55 - 56 Table 6 Scarce Vc54 Rare & Scarce taxa recorded between 2000 & 2017 57 - 59 Table 7 Rare 60 - 61 Table 8 Scarce Natives & Archaeophytes extinct & thought to be extinct in Vc54 62 - 64 Table 9 Extinct Plant list for Vice-county 54, North Lincolnshire The main table details all the Vascular Plant & Stonewort taxa with records on the MapMate botanical database for Vc54 at the end of January 2017. The table comprises: Column 1 Taxon and Authority 2 Common Name 3 Total number of records for the taxon on the database at 31/01/2017 4 Year of first record 5 Year of latest record 6 Number of hectads with records before 1/01/2000 7 Number of hectads with records between 1/01/2000 & 31/01/2017 8 Number of tetrads with records between 1/01/2000 & 31/01/2017 9 Comment & Conservation status of the taxon in Vc54 10 Conservation status of the taxon in the UK A hectad is a 10km. -

F3.1B Temperate Rubus Scrub
European Red List of Habitats - Heathland Habitat Group F3.1b Temperate Rubus scrub Summary This habitat comprises deciduous or sometimes evergreen subscrub dominated by various Rubus spp. mostly in the Atlantic and subatlantic regions, and elsewhere in locally cool conditions. It develops in ungrazed open landscapes and forest clear-cuts as part of succession, but can be a more or less persistent features of cultural landscapes such as forest margins, as hedgerows and along road verges. There is great genetic diversity among the Rubus itself (including many endemics) and the associated floras can be varied. The most important threats are related to intensification of the rural landscape, like removal of hedges, eutrophication and spread of invasive species and the habitat is replaced by scrub and forest in succession. Maintaining the diversity and dynamism of lowland landscapes is essential for conservation. Synthesis The data for assessment of trends in extent cover less than 40% of the area of the type. Although the provided data for some of the Red List criteria indicate a Least Concern status for the habitat, the overall conclusion is Data Deficient, because of the many knowledge gaps, especially for trends in quantity. Overall Category & Criteria EU 28 EU 28+ Red List Category Red List Criteria Red List Category Red List Criteria Data Deficient - Data Deficient - Sub-habitat types that may require further examination As the species composition differs largely in different regions, due to many regional endemic Rubus species, many different subhabitats may be distinguished that need further examination. In general relatively stable, "old" stands (like in forest edges or stands on former old forest localities) and stands are relatively richer in endemics than more recently developed stands developing in heathlands. -

Retamoid Scrubs of the Cytisetea Scopario-Striati in the Netherlands: a New Approach to Classify Marginal Associations
©Floristisch-soziologische Arbeitsgemeinschaft; www.tuexenia.de; download unter www.zobodat.at Tuexenia 37: 143–161. Göttingen 2017. doi: 10.14471/2016.37.022, available online at www.tuexenia.de Retamoid scrubs of the Cytisetea scopario-striati in the Netherlands: a new approach to classify marginal associations Rutenstrauchgebüsche der Cytisetea scopario-striati in den Niederlanden: ein neuer Ansatz zur Klassifikation von Marginalassoziationen Rense Haveman1, *, Iris de Ronde1 & Joop H.J. Schaminée2 1Central Government Real Estate Agency, Ministry of the Interior and Kingdom Relations, P.O. Box 47, 6700 AA Wageningen, The Netherlands; 2Alterra-WUR, Wageningen University and Research, P.O. Box 47, 6700 AA Wageningen, The Netherlands *Corresponding author, e-mail: [email protected] Abstract Marginal associations, i.e. floristically impoverished associations at the margin of the distribution area of a higher syntaxon, form a problem in vegetation classification, because true character species are lacking. We propose a new approach for the classification of such marginal associations, making use of the notion of 'chorological tension zones'. In the absence of true character species, the species from other syntaxa of the same formation can be used as such. Our proposal is to use the species group from every formation-true class only once within every marginal alliance, to limit the number of possi- ble marginal associations. This approach is illustrated in a classification of the retamoid thickets in the Netherlands. On the basis of a numerical-subjective classification of the relevant species in the scrub layer and the evaluation of relevant literature, we conclude that the broom and gorse thickets in the Netherlands can be assigned to the Cytisetea scopario-striati Rivas-Mart. -

Federal Register/Vol. 85, No. 130/Tuesday, July 7, 2020/Rules
Federal Register / Vol. 85, No. 130 / Tuesday, July 7, 2020 / Rules and Regulations 40571 § 1650.1 Definitions. clarifies confusing or contradictory revises the definitions of other terms * * * * * language in the existing regulations. The used in the regulations to provide (b) * * * revisions are expected to reduce trade greater clarity for regulated entities. Required beginning date means the burden associated with interstate seed Other revisions in this rule update the required beginning date as defined in commerce and encourage compliance seed labeling, testing, and certification Internal Revenue Code section 401(a)(9) with State and Federal laws. requirements to reflect revised and the regulations and guidance DATES: Effective August 6, 2020. terminology, as well as the evolution of promulgated thereunder. FOR FURTHER INFORMATION CONTACT: industry practices. Finally, this rule * * * * * Ernest Allen, Director, Seed Regulatory makes several revisions of an and Testing Division, Science and administrative nature to correct PART 1651—DEATH BENEFITS Technology Program, AMS, USDA; 801 misspellings and other errors in the Summit Crossing Place, Suite C, regulations. Specific revisions are ■ 6. The authority citation for Part 1651 Gastonia, NC 28054, USA; telephone: described below. continues to read as follows: 704–810–8884; email Ernest.Allen@ Revisions Authority: 5 U.S.C. 8424(d), 8432d, usda.gov. 8432(j), 8433(e), 8435(c)(2), 8474(b)(5) and Nomenclature SUPPLEMENTARY INFORMATION: The FSA 8474(c)(1). (7 U.S.C. 1551–1611) regulates interstate The regulations specify the kinds of ■ 7. Amend § 1651.1(b) by revising the commerce of planting seeds for agricultural and vegetable seed that are definition for ‘‘Required beginning agricultural and gardening purposes. -

Vegetation Classification As a Mirror of Evolution? Thoughts on the Syntaxonomy and Management of Bramble Scrubs of the Prunetalia (Rhamno-Prunetea)
Biologia (2019) 74:395–404 https://doi.org/10.2478/s11756-019-00199-x ORIGINAL ARTICLE Vegetation classification as a mirror of evolution? Thoughts on the syntaxonomy and management of bramble scrubs of the Prunetalia (Rhamno-Prunetea) Rense Haveman1,2 & Iris de Ronde1 Received: 3 October 2018 /Accepted: 25 January 2019 /Published online: 15 March 2019 # The Author(s) 2019 Abstract In Germany and the Netherlands, many bramble scrub associations are distinguished on the basis of the occurrence of Rubus species. The associations belonging to the Prunetalia spinosae Tüxen 1952 are usually assigned to the Pruno-Rubion radulae Weber Osnabr Naturwiss Mitt 3:143–150, 1974, but published tables show inconsistencies in the occurrence of alliance character species. In this paper, we compare synoptic tables from different sources from the Netherlands and Germany. From this comparison, it is concluded that the associations can be divided over two alliances, the Pruno-Rubion radulae in central Europe, and the Pruno-Rubion sprengelii Weber Osnabr Naturwiss Mitt 3:143–150, 1974 in northwest Europe (excluding the UK). The differential species of both these alliances coincide to a considerable degree with the indicator species of the phyto- geographical Rubus territories as defined by Haveman et al. (J Biogeogr 43:1360-1371, 2016). As can be deduced from recent molecular studies (Sochor et al. Mol Phylogenet Evol 89:13-27, 2015), these territories have an evolutionary background. This is an effect of the unsaturated distribution areas of a large portion of the very young Rubus agamospecies. The same holds true for the two alliances: although they have a different ecology, we argue that their current distribution areas are not a reflection of this ecology, but both their ecology and distribution area are caused by different evolutionary developments. -

Steciana Doi:10.12657/Steciana.020.025 ISSN 1689-653X
2016, Vol. 20(4): 239–249 Steciana doi:10.12657/steciana.020.025 www.up.poznan.pl/steciana ISSN 1689-653X RUBUS FLORA OF THE DUROWO FOREST DISTRICT (NORTHERN WIELKOPOLSKA, POLAND) Monika Gapińska, piotr kosiński M. Gapińska, P. Kosiński, Department of Botany, Poznań University of Life Sciences, Wojska Polskiego 71 C, 60-625 Poznań, Poland. P. Kosiński, Institute of Dendrology, Polish Academy of Sciences, Parkowa 5, PL-62-035 Kórnik, Poland, e-mail: [email protected] (Received: December 1, 2016. Accepted: December 20, 2016) abstract. The Rubus flora of northern Wielkopolska (Greater Poland) is not yet fully discovered, but the re- cent studies on the genus Rubus suggest that it is clearly not as rich as in case of southern Wielkopolska. The results of the field observations show that the area of the Durowo Forest District contains 14 representa- tives of the genus Rubus, out of which the presence of four taxa has already been reported and confirmed. Only Rubus czarnunensis, species endemic to Poland and mentioned in this area in the early 20th century, was not rediscovered. The presence of two species, previously unknown in this area, R. nemoralis and R. seeber gensis, have shifted their range limits by 16 km and 30 km to the north, respectively. A statistically signifi- cant higher number of bramble species occurred in pine forest growing on fertile deciduous forest habitats. key words: chorology, geographical distribution, range limits, occurrence condition INTRODUCTION the region, where a total of 54 species of the genus Rubus were observed (Maliński 2001). The Rubus In Poland, there is a comprehensive elaboration on flora of northern Wielkopolska appears to be dis- the taxonomy and chorology of the genus Rubus by tinctly less numerous: the herbarium collections Zieliński (2004). -

Chapter 1 Wild Plant Genetic Resources in North America
Published by: Springer Nature Switzerland AG 2018 Citation: Greene SL, Khoury CK, and Williams KA (2018). “Wild plant genetic resources in North America: an overview”. In: Greene SL, Williams KA, Khoury CK, Kantar MB, and Marek LF, eds., North American Crop Wild Relatives, Volume 1: Conservation Strategies. Springer, doi: 10.1007/978-3-319-95101-0_1. https://doi.org/10.1007/978-3-319-95101-0_1 https://link.springer.com/chapter/10.1007%2F978-3-319-95101-0_1 Chapter 1 Wild plant genetic resources in North America: an overview Stephanie L. Greene*, Colin K. Khoury, and Karen A. Williams Stephanie L. Greene*, USDA, Agricultural Research Service, Center for Agricultural Resources Research, National Laboratory for Genetic Resources Preservation, Fort Collins, CO, USA, [email protected] *corresponding author Colin K. Khoury, USDA, Agricultural Research Service, Center for Agricultural Resources Research, National Laboratory for Genetic Resources Preservation, Fort Collins, CO, USA and International Center for Tropical Agriculture (CIAT), Cali, Colombia [email protected], [email protected] Karen A. Williams, USDA, Agricultural Research Service, National Germplasm Resources Laboratory, Beltsville Agricultural Research Center ,Beltsville, MD, USA, [email protected] Abstract North America, including Canada, Mexico and the United States, is rich in plant species used by humans in both ancient and modern times. A select number of these have become globally important domesticated crops, including maize, beans, cotton, and sunflower. Many other native and also naturalized species have potential for use, either directly or as genetic resources for breeding agricultural crops. However, despite increasing recognition of their potential value, deficiencies in information, conservation, and access to the diversity in these plants hinder their further use. -

Agricultural Marketing Service Technology Program, AMS, USDA; 801 Agricultural and Vegetable Seed That Are Summit Crossing Place, Suite C, Subject to Regulation
4603 Proposed Rules Federal Register Vol. 85, No. 17 Monday, January 27, 2020 This section of the FEDERAL REGISTER rule will be included in the rulemaking evolution of industry practices. Finally, contains notices to the public of the proposed record and will be made available to the AMS is proposing several revisions of issuance of rules and regulations. The public. Please be advised that the an administrative nature to correct purpose of these notices is to give interested identity of the individuals or entities misspellings and other errors in the persons an opportunity to participate in the submitting comments will be made regulations. Specific proposals are rule making prior to the adoption of the final rules. public on the internet at the address addressed below. provided above. Proposals FOR FURTHER INFORMATION CONTACT: DEPARTMENT OF AGRICULTURE Ernest Allen, Director, Seed Regulatory Nomenclature and Testing Division, Science and The regulations specify the kinds of Agricultural Marketing Service Technology Program, AMS, USDA; 801 agricultural and vegetable seed that are Summit Crossing Place, Suite C, subject to regulation. AMS proposes to 7 CFR Parts 201 and 202 Gastonia, NC 28054, USA; telephone: revise the list of agricultural seed [Doc. No. AMS–ST–19–0039] 704–810–8884; email Ernest.Allen@ covered by the regulation in § 201.2(h) usda.gov. by adding camelina, radish, and teff to Revisions to the Federal Seed Act SUPPLEMENTARY INFORMATION: The FSA the list. The proposed revisions would Regulations (7 U.S.C. 1551–1611) regulates interstate add radish to the list of seed kinds for and foreign commerce of planting seeds which the variety is required on the AGENCY: Agricultural Marketing Service, for agricultural and gardening purposes. -

Rare Plant Register
1 BSBI RARE PLANT REGISTER Berkshire & South Oxfordshire V.C. 22 MICHAEL J. CRAWLEY FRS UPDATED APRIL 2005 2 Symbols and conventions The Latin binomial (from Stace, 1997) appears on the left of the first line in bold, followed by the authority in Roman font and the English Name in italics. Names on subsequent lines in Roman font are synonyms (including names that appear in Druce’s (1897) or Bowen’s (1964) Flora of Berkshire that are different from the name of the same species in Stace). At the right hand side of the first line is a set of symbols showing - status (if non-native) - growth form - flowering time - trend in abundance (if any) The status is one of three categories: if the plant arrived in Britain after the last ice age without the direct help of humans it is defined as a native, and there is no symbol in this position. If the archaeological or documentary evidence indicates that a plant was brought to Berkshire intentionally of unintentionally by people, then that species is an alien. The alien species are in two categories ● neophytes ○ archaeophytes Neophytes are aliens that were introduced by people in recent times (post-1500 by convention) and for which we typically have precise dates for their first British and first Berkshire records. Neophytes may be naturalized (forming self-replacing populations) or casual (relying on repeated introduction). Archaeophytes are naturalized aliens that were carried about by people in pre-historic times, either intentionally for their utility, or unintentionally as contaminants of crop seeds. Archaeophytes were typically classified as natives in older floras. -
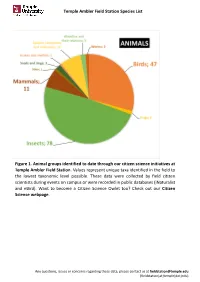
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Gervuogių Vaisių Antocianinų Sudėties Tyrimas Efektyviosios Skysčių Chromatografijos (Esc) Metodu
LIETUVOS SVEIKATOS MOKSLŲ UNIVERSITETAS FARMACIJOS FAKULTETAS FARMAKOGNOZIJOS KATEDRA ALIONA GRIŠKEVIČ GERVUOGIŲ VAISIŲ ANTOCIANINŲ SUDĖTIES TYRIMAS EFEKTYVIOSIOS SKYSČIŲ CHROMATOGRAFIJOS (ESC) METODU Magistro baigiamasis darbas Darbo vadovas: lekt. dr. D. Burdulis KAUNAS, 2016 2 LIETUVOS SVEIKATOS MOKSLŲ UNIVERSITETAS FARMACIJOS FAKULTETAS FARMAKOGNOZIJOS KATEDRA TVIRTINU: Farmacijos fakulteto dekanas Vitalis Briedis Data GERVUOGIŲ VAISIŲ ANTOCIANINŲ SUDĖTIES TYRIMAS EFEKTYVIOSIOS SKYSČIŲ CHROMATOGRAFIJOS (ESC) METODU Magistro baigiamasis darbas Darbo vadovas: lekt. dr. Deividas Burdulis Data Recenzentas: Darbą atliko: Magistrantė Aliona Griškevič Data Data KAUNAS, 2016 3 TURINYS SANTRAUKA .................................................................................................................................... 5 SUMMARY ........................................................................................................................................ 6 SANTRUMPOS .................................................................................................................................. 7 ĮVADAS ............................................................................................................................................. 8 DARBO TIKSLAS IR UŢDAVINIAI ............................................................................................... 10 1. LITERATŪROS APŢVALGA .................................................................................................. 11 1.1 Gervuogių genties (Rubus L.)