Immune Checkpoint Molecules in Natural Killer Cells As Potential Targets for Cancer Immunotherapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IL-15 Stimulation with TIGIT Blockade Reverses CD155-Mediated NK Cell
Author Manuscript Published OnlineFirst on June 26, 2020; DOI: 10.1158/1078-0432.CCR-20-0575 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. IL-15 stimulation with TIGIT blockade reverses CD155-mediated NK cell dysfunction in melanoma Joe-Marc Chauvin1, Mignane Ka1, Ornella Pagliano1, Carmine Menna1, Quanquan Ding1, Richelle DeBlasio1, Cindy Sanders1, Jiajie Hou2, Xian-Yang Li3, Soldano Ferrone4, Diwakar Davar1, John M. Kirkwood1, Robert Johnston5, Alan J. Korman5, Mark J. Smyth3, and Hassane M. Zarour1,6. 1Department of Medicine and Division of Hematology/Oncology, University of Pittsburgh, School of Medicine, Pittsburgh, PA 15213, USA. 2Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China. 3Immunology in Cancer and Infection Laboratory, QIMR Berghofer Medical Research Institute, Queensland, Australia. 4Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA. 5Biologics Discovery California, Bristol-Myers Squibb, Redwood City, CA 94063, USA. 6Department of Immunology, University of Pittsburgh, School of Medicine, Pittsburgh, PA 15213, USA. Running title: IL-15 and TIGIT blockade reverse NK dysfunction in melanoma Keywords: Melanoma, Immunotherapy, TIGIT, IL-15, NK cells This work was supported by NIH/NCI grants R01CA228181 and R01CA222203 (to HMZ), a research grant by Bristol-Myers Squibb BMS (to HMZ), a cancer vaccine collaborative clinical strategy team grant (to HMZ), and NCI grant P50CA121973 (to 1 Downloaded from clincancerres.aacrjournals.org on September 25, 2021. © 2020 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 26, 2020; DOI: 10.1158/1078-0432.CCR-20-0575 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Selectins in Cancer Immunity
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Selectins in cancer immunity Borsig, Lubor Abstract: Selectins are vascular adhesion molecules that mediate physiological responses such as inflam- mation, immunity and hemostasis. During cancer progression, selectins promote various steps enabling the interactions between tumor cells and the blood constituents, including platelets, endothelial cells and leukocytes. Selectins are carbohydrate-binding molecules that bind to sialylated, fucosylated glycan structures. The increased selectin ligand expression on tumor cells correlates with enhanced metastasis and poor prognosis for cancer patients. While, many studies focused on the role of selectin as a mediator of tumor cell adhesion and extravasation during metastasis, there is evidence for selectins to activate signaling cascade that regulates immune responses within a tumor microenvironment. L-Selectin binding induces activation of leukocytes, which can be further modulated by selectin-mediated interactions with platelets and endothelial cells. Selectin ligand on leukocytes, PSGL-1, triggers intracellular signaling in leukocytes that are induced through platelet’s P-selectin or endothelial E-selectin binding. In this review, I summarize the evidence for selectin-induced immune modulation in cancer progression that represents a possible target for controlling tumor immunity. DOI: https://doi.org/10.1093/glycob/cwx105 Posted at the Zurich Open Repository and -

Molecular and Clinical Characterization of LAG3 in Breast Cancer Through 2994 Samples
Molecular and Clinical Characterization of LAG3 in Breast Cancer Through 2994 Samples Qiang Liu Chinese Academy of Medical Sciences & Peking Union Medical College Yihang Qi ( [email protected] ) Chinese Academy of Medical Sciences and Peking Union Medical College https://orcid.org/0000-0001- 7589-0333 Jie Zhai Chinese Academy of Medical Sciences & Peking Union Medical College Xiangyi Kong Chinese Academy of Medical Sciences & Peking Union Medical College Xiangyu Wang Chinese Academy of Medical Sciences & Peking Union Medical College Yi Fang Chinese Academy of Medical Sciences & Peking Union Medical College Jing Wang Chinese Academy of Medical Sciences & Peking Union Medical College Research Keywords: Cancer immunotherapy, CD223, LAG3, Immune response, Inammatory activity Posted Date: June 19th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-36422/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/33 Abstract Background Despite the promising impact of cancer immunotherapy targeting CTLA4 and PD1/PDL1, a large number of cancer patients fail to respond. LAG3 (Lymphocyte Activating 3), also named CD233, is a protein Coding gene served as alternative inhibitory receptors to be targeted in the clinic. The impact of LAG3 on immune cell populations and co-regulation of immune response in breast cancer remained largely unknown. Methods To characterize the role of LAG3 in breast cancer, we investigated transcriptome data and associated clinical information derived from a total of 2994 breast cancer patients. Results We observed that LAG3 was closely correlated with major molecular and clinical characteristics, and was more likely to be enriched in higher malignant subtype, suggesting LAG3 was a potential biomarker of triple-negative breast cancer. -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Cancer Immunology, Immunotherapy Editor-In-Chief: H
Cancer Immunology, Immunotherapy Editor-in-Chief: H. Dong ▶ 94% of authors who answered a survey reported that they would definitely publish or probably publish in the journal again Since its inception in 1976, Cancer Immunology, Immunotherapy (CII) has reported significant advances in the field of tumor immunology. The journal serves as a forum for new concepts and advances in basic, translational, and clinical cancer immunology and immunotherapy. CII is keen to publish broad-ranging ideas and reviews, results which extend or challenge established paradigms, as well as negative studies which fail to reproduce experiments that support current paradigms, and papers that do succeed in reproducing others’ results in different contexts. Cll is especially interested in papers describing clinical trial designs and outcome regardless of whether they met their designated endpoints or not, and particularly those shedding light on immunological mechanisms. CII is affiliated with the Association for Cancer Immunotherapy (CIMT), Canadian Cancer 12 issues/year Immunotherapy Consortium (CCIC), The Japanese Association for Cancer Immunology (JACI), Network Italiano per la Bioterapia dei Tumori (NIBIT),and Sociedad Española de Inmunologia- Electronic access Grupo Española de InmunoTerapia (SEI-GEIT). ▶ link.springer.com CIMT: http://www.cimt.eu/home Subscription information CCIC: http://www.immunotherapycancer.ca/ ▶ springer.com/librarians JACI: http://www.jaci.jp/eng/index.html NIBIT: http://www.nibit.org/index.php SEI-GEIT: http://www.inmunologia.org/grupos/home.php? UpOm5=M&Upfqym5uom=GK CITIM: http://www.canceritim.org Impact Factor: 6.968 (2020), Journal Citation Reports® On the homepage of Cancer Immunology, Immunotherapy at springer.com you can ▶ Sign up for our Table of Contents Alerts ▶ Get to know the complete Editorial Board ▶ Find submission information. -

Prospects for NK Cell Therapy of Sarcoma
cancers Review Prospects for NK Cell Therapy of Sarcoma Mieszko Lachota 1 , Marianna Vincenti 2 , Magdalena Winiarska 3, Kjetil Boye 4 , Radosław Zago˙zd˙zon 5,* and Karl-Johan Malmberg 2,6,* 1 Department of Clinical Immunology, Doctoral School, Medical University of Warsaw, 02-006 Warsaw, Poland; [email protected] 2 Department of Cancer Immunology, Institute for Cancer Research, Oslo University Hospital, 0310 Oslo, Norway; [email protected] 3 Department of Immunology, Medical University of Warsaw, 02-097 Warsaw, Poland; [email protected] 4 Department of Oncology, Oslo University Hospital, 0310 Oslo, Norway; [email protected] 5 Department of Clinical Immunology, Medical University of Warsaw, 02-006 Warsaw, Poland 6 Center for Infectious Medicine, Department of Medicine Huddinge, Karolinska Institutet, Karolinska University Hospital, 141 86 Stockholm, Sweden * Correspondence: [email protected] (R.Z.); [email protected] (K.-J.M.) Received: 15 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Sarcomas are a group of aggressive tumors originating from mesenchymal tissues. Patients with advanced disease have poor prognosis due to the ineffectiveness of current treatment protocols. A subset of lymphocytes called natural killer (NK) cells is capable of effective surveillance and clearance of sarcomas, constituting a promising tool for immunotherapeutic treatment. However, sarcomas can cause impairment in NK cell function, associated with enhanced tumor growth and dissemination. In this review, we discuss the molecular mechanisms of sarcoma-mediated suppression of NK cells and their implications for the design of novel NK cell-based immunotherapies against sarcoma. -

5.01.591 Immune Checkpoint Inhibitors
MEDICAL POLICY – 5.01.591 Immune Checkpoint Inhibitors Effective Date: Sept. 1, 2021 RELATED POLICIES/GUIDELINES: Last Revised: Aug. 10, 2021 5.01.543 General Medical Necessity Criteria for Companion Diagnostics Related Replaces: N/A to Drug Approval 5.01.589 BRAF and MEK Inhibitors Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Chemotherapy, often called chemo, is cancer treatment that uses drugs. Radiation and surgery treat one area of cancer. But chemo usually travels through the bloodstream to treat the whole body. Treating the whole body is called a systemic treatment. Immunotherapy is a new type of cancer treatment that helps the body’s immune cells fight the cancer more effectively. Cancer cells sometimes “hide” from the body’s cells that are designed to search for cells that don’t belong, like cancer cells or bacteria. Immune checkpoint inhibitors are drugs that block the way that cancer cells do this and so help the immune cells find them so they can be stopped. It is one of the ways we can make the environment less friendly to the cancer and slow its growth. Current immunotherapy drugs are complex molecules that must be given through a vein (intravenous). In the future, some may be given by a shot (injection) the patient could inject without help. This policy gives information about immunotherapy drugs and the criteria for when they may be medically necessary. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. -
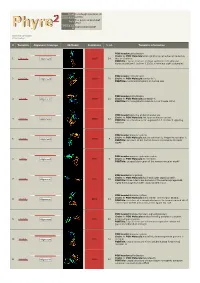
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

LAG-3: from Molecular Functions to Clinical Applications
Open access Review J Immunother Cancer: first published as 10.1136/jitc-2020-001014 on 13 September 2020. Downloaded from LAG-3: from molecular functions to clinical applications Takumi Maruhashi , Daisuke Sugiura , Il- mi Okazaki , Taku Okazaki To cite: Maruhashi T, Sugiura D, ABSTRACT (PD-1) and cytotoxic T lymphocyte antigen Okazaki I, et al. LAG-3: from To prevent the destruction of tissues owing to excessive 4 (CTLA-4) significantly improved the molecular functions to clinical and/or inappropriate immune responses, immune outcomes of patients with diverse cancer applications. Journal for cells are under strict check by various regulatory ImmunoTherapy of Cancer types, revolutionizing cancer treatment. The mechanisms at multiple points. Inhibitory coreceptors, 2020;8:e001014. doi:10.1136/ success of these therapies verified that inhib- including programmed cell death 1 (PD-1) and cytotoxic jitc-2020-001014 itory coreceptors serve as critical checkpoints T lymphocyte antigen 4 (CTLA-4), serve as critical checkpoints in restricting immune responses against for immune cells to not attack the tumor Accepted 29 July 2020 self- tissues and tumor cells. Immune checkpoint inhibitors cells as well as self-tissues. However, response that block PD-1 and CTLA-4 pathways significantly rates are typically lower and immune-related improved the outcomes of patients with diverse cancer adverse events (irAEs) are also observed in types and have revolutionized cancer treatment. However, patients administered with immune check- response rates to such therapies are rather limited, and point inhibitors. This is indicative of the immune-rela ted adverse events are also observed in a continued need to decipher the complex substantial patient population, leading to the urgent need biology of inhibitory coreceptors to increase for novel therapeutics with higher efficacy and lower response rates and prevent such unwanted toxicity. -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea.