5 XII December 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Census Handbook, Hyderabad, Part XIII a & B, Series-2
CENSUS OF INDIA 1981 SERIES 2 ANDHRA PRADESH DISTRICT CENSUS. HANDBOOK HYDERABAD PARTS XIII-A & B VILLAGE & TOWN DIRECTORY VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT S. S. JAYA RAO OF THE INDIAN ADMINISTRATIVE SERVICE DIRECTOR OF CENSUS OPERATIONS ANDHRA PRADESH PUBLISHED BY THE GOVERNMENT OF ANDHRA PRADESH 1987 ANDHRA PRADESH LEGISLATURE BUILDING The motif presented on the cover page represents the new Legislature building of Andhra Pradesh State located in the heart of the capital city of Hyderabad. August, 3rd, 1985 is a land mark in the annals of the Legislature of Anohra Pradesh on which day the Prime Minister, Sri Rajiv Gandhi inaugu rated the Andhra Pradesh Legislacure Build ings. The newly constructed Assembly Build ing of Andhra Pradesh is located in a place adorned by thick vegitation pervading with peaceful atmosphere with all its scenic beauty. It acquires new dimensions of beauty, elegance and modernity with its gorgeous and splen did constructions, arches, designs, pillars of various dImensions, domes etc. Foundation stone for this new Legislature Building was laid by the then Chief Minister, Dr. M. Chenna Reddy on 19th March, 1980. The archilecture adopted for the exterior devation to the new building is the same as that of the old building, leaving no scope for differentiation between the two building~. The provision of detached round long columns under the arches add more beauty to the building. The building contains modern amenities such as air-connitioning, interior decoration and reinforced sound system. There is a provision for the use of modc:rn sophisticated electronic equipment for providing audio-system. -

A S Rao Nagar Branch Mgl 3618 Rajulal Choudary 1-30/60/7
A S RAO NAGAR BRANCH A S RAO NAGAR BRANCH A S RAO NAGAR BRANCH MGL 3618 MGL 3619 MSGL 259 RAJULAL CHOUDARY JAGADISH CHOUDARY P.AMARNATH 1-30/60/7, HNO 1-1-30/171 S/O P.KESHAVULU, G.R. REDDY NAGAR, G.R.REDDY NAGAR, H.NO: 1-11-80, SVS NAGAR, DAMMAIGUDA KAPRA KEESARA RANGA REDDY KUSHAIGUDA, HYD 500062 HYD 500 HYD 500062 A S RAO NAGAR BRANCH A S RAO NAGAR BRANCH ABIDS BRANCH MSGL 260 MSGL 261 MGL 103 A PARTHA SARATHI A PARTHA SARATHI NISHA JAJU HNO 1-86 RAMPALLI DAYARA HNO 1-86 RAMPALLI DAYARA 3-4-516 KEESARA MANDAL RR DIST KEESARA MANDAL RR DIST BARKATPURA HYDERABAD HYDERABAD OPP ARUNA STUDIO HYD 501301 HYD 501301 HYD 500029 ABIDS BRANCH ABIDS BRANCH ABIDS BRANCH MGL 109 MGL 110 MGL 111 MADHAVI TOPARAM MADHAVI TOPARAM MADHAVI TOPARAM FLAT NO 401 1-5-808/123/34/40 FLAT NO 401 1-5-808/123/34/40 FLAT NO 401 1-5-808/123/34/40 HANUMAN ENCLAVE HANUMAN ENCLAVE HANUMAN ENCLAVE MUSHEERABAD NEAR MUSHEERABAD NEAR MUSHEERABAD NEAR EK MINAR ZAMISTANPOOR EK MINAR ZAMISTANPOOR EK MINAR ZAMISTANPOOR HYDERABAD HYDERABAD HYDERABAD HYD 500020 HYD 500020 HYD 500020 ABIDS BRANCH ABIDS BRANCH ABIDS BRANCH MGL 115 MGL 116 MGL 118 SHIVA PRASAD MUTHINENI ETIKAALA VENKATESH KOTHAPALLI SATYANARAYANA 2-2-1167/11/A/3 TILAK NAGAR 5-1-74-VEERA BHRAMENDRA 3-5-1093/10/1 MUSHEERABAD NEW SWAMY NAGAR NEAR KAALI VENKATESHWARA COLONY NALLAKUNTA MANDHIR NARAYANGUDA HYDERABAD HYDERABAD BANDLAGUDA JAGIR HYD 500029 HYD 500044 RAJENDRANAGAR HYD 500086 ABIDS BRANCH ABIDS BRANCH ABIDS BRANCH MGL 119 MGL 129 MGL 130 KOTHAPALLI SATYANARAYANA HANSRAJ NAGIRI SOUJANYA 3-5-1093/10/1 5-1-51,SARDARJI -

Government of Telangana Commercial Taxes Department
GOVERNMENT OF TELANGANA COMMERCIAL TAXES DEPARTMENT TGST Notification No.19/2019 CST’S Ref No.C/736/2016 Dated 17-05-2019 Sub: Notification of Tax persons to the respective jurisdictions Ref : 1. GO Ms.No.145 Dated. 02-08-2018 2. TGST Notification No.7/2019 dt.26-02-2019 In the ref. cited GO, Government has rationalized the circles and enhanced the circles from 91 to 100 in the Commercial Taxes Department on administrative exigency within the available cadre strength and also the Government has agreed for re-designation of Large Tax Payer Units (LTU) to Strategic Tax Payer Units (STU). In the ref. 1st cited GO, the undersigned is directed to notify the Tax persons to the respective jurisdictions as per the rationalisation in the Commercial Taxes Department. Tax persons are allocated to the respective jurisdictions by IIT Hyderabad. Vide ref. 2nd cited, individual tax payers are allocated to the respective jurisdictions. In continuation and in modification to the said Notification vide ref. 2nd cited, the following latest lists of individual tax payers are allocated to the respective jurisdictions by IIT Hyderabad. 1. State Jurisdiction Taxpayers (S2S, N2S, C2S) 2. Centre Jurisdiction Taxpayers (S2C) 3. Centre Jurisdiction Taxpayers (C2C, N2C) 4. Not Migrated from VATIS Taxpayers The above lists are based on information of Taxpayers as on 15-03- 2019. Abstract of latest allocation of Tax payers is appended to this Notification. Soft copies of latest allocation of individual Tax payers to the respective jurisdictions (Four lists as stated above) will be attached along Page 1 of 8 with the Notification under the tab ‘GST Circulars’ displayed in the website www.tgct.gov.in as the file size is 41MB. -

Protests Across India Today
c m y k c m y k THE LARGEST CIRCULATED ENGLISH DAILY IN SOUTH INDIA HYDERABAD I THURSDAY I 19 DECEMBER 2019 WEATHER O WORLD 8 TABLOID Max: 27.8 C | SPORTS 15 Min: 18.2OC | RH: 55% Trump faces India beat Windies to Smart ways of Rainfall: Nil crucial vote level One-Day series cutting costs Forecast: Cloudy sky. Misty morning. Max/Min temp. 28/18ºC deccanchronicle.com, facebook.com/deccannews, twitter.com/deccanchronicle, google.com/+deccanchronicle Vol. 82 No. 349 Established 1938 | 40 PAGES | `6.00 KCR WANTS TO HOLD CIVIC POLLS Mistry reinstated as Tata Group head Rapist’s plea BY SANKRANTI RAVI RANJAN PRASAD | FC closed in the red. company from a public MUMBAI, DEC. 18 Mr Mistry was removed firm and directed the S.N.C.N. ACHARYULU | DC as the chairman on October Registrar of Companies to for SC review HYDERABAD, DEC. 18 In one of the biggest corpo- 24, 2016 after helming the correct its record showing rate developments of 2019, Tata Group for over four Tata Sons as a public com- Seeing an unexpected the National Company Law years. He was appointed in pany. NCLAT’s 172-page political advantage in the Appellate Tribunal (NCL- 2012, after Ratan Tata order also directed Ratan controversial Citizenship AT) has restored Cyrus retired. He was the sixth Tata and nominees of the turned down Amendment Act (CAA), Mistry as executive chair- chairman and only the sec- Tata Trust to desist from Telangana Chief Minister man of the Tata Group and ond, after Nowroji Saklatw- any decision that requires Court gives 7 days to Nirbhaya K. -

Government of India Ministry of Skill Development and Entrepreneurship
GOVERNMENT OF INDIA MINISTRY OF SKILL DEVELOPMENT AND ENTREPRENEURSHIP LOK SABHA UNSTARRED QUESTION NO. 1413 TO BE ANSWERED ON 05.03.2018 INTEGRATION OF ENTREPRENEURSHIP EDUCATION 1413. SHRI A.P. JITHENDER REDDY: Will the Minister of SKILL DEVELOPMENT AND ENTREPRENEURSHIP be pleased to state: (a) the details of the colleges of Telangana which have been identified for integration of entrepreneurship education along with mainstream education under the National Policy for Skill Development and Entrepreneurship 2015; (b) the details of the progress made for the integration and anticipated time by which all the identified colleges would be integrated; and (c) whether any Nodal Entrepreneurship Hubs are being set up in Telangana and if so, the details thereof? ANSWER MINISTER IN THE MINISTRY OF SKILL DEVELOPMENT AND ENTREPRENEURSHIP (SHRI DHARMENDRA PRADHAN) (a) In order to achieve the targets and objectives of National Policy for Skill Development & Entrepreneurship 2015 Ministry of Skill Development & Entrepreneurship (MSDE) has launched its flagship scheme Pradhan Mantri Yuva Udyamita Vikas Abhiyan (PM-YUVA) on 9 th November, 2016. Although the scheme was launched on 9 th November, 2016, it was awaiting for signing of implementation agreement with Wadhwani Foundation (The Knowledge Partner), which was signed on 23.02.2017. Hence, the project actually started during the financial year 2017-18. The scheme aims to create an enabling ecosystem for Entrepreneurship Development through entrepreneurship education and training across the country in select Institutes of Higher Learning (Universities, Colleges and Premier Institutes), schools, Industrial Training Centers (ITIs) and Entrepreneurship Development Centers (EDCs) for over a period of five years (2016-17 to 2020-21). -
![[ Details of the Bidder ]](https://docslib.b-cdn.net/cover/7401/details-of-the-bidder-2127401.webp)
[ Details of the Bidder ]
GREATER HYDERABAD MUNICIPAL CORPORATION REQUEST FOR PROPOSAL DESIGN, SUPPLY, INSTALLATION, OPERATION AND MAINTANENCE OF INTERGRATED INTEGRATED SMART WASHROOMS ON DESIGN, BUILD, FINANCE OPERATE & TRANSFER (DBFOT) BASIS AT 30 LOCATIONS IN CHARMINAR ZONE, GHMC (PACKAGE No I) Proposed By: Commissioner, Greater Hyderabad Municipal Corporation Hyderabad– 500029 Prepared by Submitted by : [ details of the bidder ] --------------------------------------------------------- ----------------------------------------------------------- ------------------------------------------------------------ ------------------------------------------------------------ _____________________________________________________________________________________ Package I GHMC Charrminar ZONE – RFP BID DOCUMENT for SMART INTEGRATED TOILETS Page 1 of 80 Greater Hyderabad Municipal Corporation Government of Telangana Office of the Superintending Engineer (SWM) IV floor, CC Complex , GHMC Head Office, Tank Bund, Hyderabad – 500029 INVITATION FOR REQUEST FOR PROPOSAL (RFP) Greater Hyderabad Municipal Corporation (GHMC) invites “Request for Proposal” from Public Limited/Private Limited Companies who have adequate experience and adequate financial strength in this field for “Design, supply, installation, operation & maintenance of Integrated Smart Washrooms on Design, Finance, Build, Operate & Transfer (DBFOT) basis” at 30 Locations in CHARMINAR ZONE, GHMC (Package No I) as per the terms and conditions laid down in this RFP document. The RFP Bid Document may be downloaded from -

72J Bus Time Schedule & Line Route
72J bus time schedule & line map 72J Afzal Gunj - Bandlaguda X Road View In Website Mode The 72J bus line (Afzal Gunj - Bandlaguda X Road) has 2 routes. For regular weekdays, their operation hours are: (1) Afzal Gunj Bus Stop: 10:00 AM (2) Bandlaguda X Road: 10:00 AM Use the Moovit App to ƒnd the closest 72J bus station near you and ƒnd out when is the next 72J bus arriving. Direction: Afzal Gunj Bus Stop 72J bus Time Schedule 17 stops Afzal Gunj Bus Stop Route Timetable: VIEW LINE SCHEDULE Sunday 10:00 AM Monday 10:00 AM Old Nagole Tuesday 10:00 AM Nagole X Road Wednesday 10:00 AM New Nagole Thursday 10:00 AM Mohan Nagar Friday 10:00 AM Sbi Colony Saturday 10:00 AM Kothapet Chaitanyapuri 72J bus Info dilsukh nagar main road, Hyderābād Direction: Afzal Gunj Bus Stop Dilsukhnagar Bus Station (Opposite Side) Stops: 17 Trip Duration: 25 min T.V.Tower / Moosaram Bagh Line Summary: Old Nagole, Nagole X Road, New Nagole, Mohan Nagar, Sbi Colony, Kothapet, Chaitanyapuri, Dilsukhnagar Bus Station (Opposite Malakpet Super Bazar Side), T.V.Tower / Moosaram Bagh, Malakpet Super Bazar, Yashoda Hospital, Nalgonda X Roads, Yashoda Hospital Chaderghat, M.G.B.S., Central Bus Station (C.B.S.), Gowliguda Bus Depot, Afzalgunj Nalgonda X Roads SH19, Hyderābād Chaderghat NH163, Hyderābād M.G.B.S. Central Bus Station (C.B.S.) Maulvi Aladdin Road, Hyderābād Gowliguda Bus Depot Afzalgunj NH44, Hyderābād Direction: Bandlaguda X Road 72J bus Time Schedule 18 stops Bandlaguda X Road Route Timetable: VIEW LINE SCHEDULE Sunday 10:00 AM Monday 10:00 AM Afzalgunj NH44, Hyderābād Tuesday 10:00 AM Gowliguda Bus Depot Wednesday 10:00 AM Central Bus Station (C.B.S.) Thursday 10:00 AM Friday 10:00 AM M.G.B.S. -

Musi River Pollution on Human Anthropogenic Activities Srisailam Gogula 1 and Sunder Kumar Kolli 2* 1Department of Chemistry, Govt
Research Journal of Chemical Sciences _________________________________ ______ _____ E-ISSN 2231-606X Vol. 6(12), 18-24, December (201 6) Res. J. Chem. Sci. Effect of Musi River Pollution on Human Anthropogenic Activities Srisailam Gogula 1 and Sunder Kumar Kolli 2* 1Department of Chemistry, Govt. City College, Hyderabad, Telangana -500008 , India 2Department of Chemistry, Annamacharya Institute of Technology Sciences, Hyderabad , India [email protected] Available online at: www.isca.in, www.isca.me Received 22 nd May 2016, revised 5th December 2016, accepted 13 th December 201 6 Abstract At the present day the world is mainly focused on the depletion of the atmospheric ozone layer by environmental pollution. Environmental pollution is unfavorable alteration of our surroundings . The water is most important resource and one of the universal solvent, it is used by living organisms. The major source of water is mainly oceans, rivers, lakes, ponds and makes 65% of human body. The water is us ing for daily activities and also used for several industries may causes water pollution. In present study, an extensive investigation of physico -chemical parameters of water samples of river Musi located in Hyderabad was carried out. For this area samplin g sites were selected along the river Musi in and around Hyderabad on affected areas like Himayath Sagar-1, Langer House -2, Govt. City college-3, Nagole-4 and Peerjadiguda -5 (Ground water). Water samples were collected during a month of February 2016. The observed values of different parameters such as Colour, Odour, pH, EC, TDS, Turbidity, CO 3, HCO 3, Cl, F, NO 3, SO 4, Na, K, Ca, Mg, TH, BOD and COD of samples were indentified in different locations in and around Hyderabad city. -

9M Bus Time Schedule & Line Route
9M bus time schedule & line map 9M Falaknuma Bus Station View In Website Mode The 9M bus line (Falaknuma Bus Station) has 2 routes. For regular weekdays, their operation hours are: (1) Falaknuma Bus Station: 6:30 AM - 7:25 PM (2) Sanath Nagar Bus Depot: 7:40 AM - 8:45 PM Use the Moovit App to ƒnd the closest 9M bus station near you and ƒnd out when is the next 9M bus arriving. Direction: Falaknuma Bus Station 9M bus Time Schedule 50 stops Falaknuma Bus Station Route Timetable: VIEW LINE SCHEDULE Sunday 6:30 AM - 7:25 PM Monday 6:30 AM - 7:25 PM Sanath Nagar Bus Depot Tuesday 6:30 AM - 7:25 PM Sanath Nagar Wednesday 6:30 AM - 7:25 PM Sanath Nagar Police Station Thursday 6:30 AM - 7:25 PM Erragadda Towards Sanathnagar Friday 6:30 AM - 7:25 PM Erragadda Saturday 6:30 AM - 7:25 PM Erragadda Fci Allwyn Erragadda 9M bus Info Rythu Bazar Erragadda Direction: Falaknuma Bus Station Stops: 50 Trip Duration: 56 min Erragadda Fci Line Summary: Sanath Nagar Bus Depot, Sanath Nagar, Sanath Nagar Police Station, Erragadda ESI Towards Sanathnagar, Erragadda, Erragadda Fci, Allwyn Erragadda, Rythu Bazar Erragadda, E S I Erragadda Fci, ESI, E S I, S R Nagar Bus Stop, Sanjeeva Reddy Nagar, Maithrivanam, Ameerpet Bus S R Nagar Bus Stop Stop, Punjagutta Bus Stop, Nims, Punjagutta, Erramanzil, Khairatabad Rta, Khairatabad Bus Stop, Sanjeeva Reddy Nagar Shaadan College, Chintal Basti, Lakdikapul, Saifabad, Telephone Bhavan, Accountant General (A Maithrivanam & G) O∆ce / Birla Mandir, Assembly, Assembly, Nampally Grand Plaza, Nampally, Nampally Haj Ameerpet -
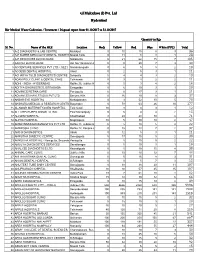
Monthly Report
GJ Multiclave (I) Pvt. Ltd Hyderabad Bio-Medical Waste Collection / Treatment / Disposal report from 01.10.2017 to 31.10.2017 Quantity in Kgs Sl. No. Name of the HCE Location Beds Yellow RedRedRed Blue White (PPC) Total 1 A-Z DIAGNOSTIC & LAB CENTRE Malakpet 0 10 15 8 3 36 2 A.P SUPER SPECIALITY DENTAL HOSPITALMasab Tank 0 11 8 3 1 23 3 A.P.REDCROSS BLOOD BANK Nallakunta 0 21 62 15 7 105 4 AAROHI BLOOD BANK Rd. No.1 Banjara Hills 0 0 20 7 3 30 5 ACCENTURE SERVICES PVT LTD. ( SEZ ) Nanakramguda 0 6 5 5 2 18 6 ACCESS DENTAL HOSPITAL Alwal 0 2 5 3 1 11 7 ACHARYA TULSI DIAGNOSTICS CENTRE Boinpally 0 4 4 3 2 13 8 ADAM POLY CLINIC & DENTAL CARE Tolichowki 0 3 4 2 2 11 9 ADHI - INDIA - HYDERABAD Rd.No.36, Jubilee Hills 0 5 3 4 2 14 10 ADITYA DIAGNOSTICS, ERRAGADA Erragadda 0 6 10 3 1 20 11 ADVANCE RETINA CARE Panjagutta 0 0 17 3 1 21 12 ADVANCES HAIR STUDIO PVT LTD Banjara Hills 0 16 10 8 3 37 13 AKBAR EYE HOSPITAL Mehdipatnam 0 3 52 10 5 70 14 AKSHARA MEDICAL & RESEARCH CENTREBegumpet (Ankura) 0 78 63 26 10 177 15 AL-NOOR MATERNITY&GEN HOSPITAL Tolichowki 10 8 0 3 1 12 16 ALL CREATURES ANIMAL CLINIC East Marredpally 0 5 4 4 2 15 17 ALLWIN HOSPITAL Khairthabad 8 29 28 10 4 71 18 ALPHA HOSPITAL Moghalpura 10 5 30 10 2 47 19 AMAR IMMUNODIAGNOSTICS PVT LTD Rd.No.18, Jubilee Hills 0 5 10 5 2 22 20 AMBROSIA CLINIC Rd.No.12, Banjara Hills 0 15 13 7 2 37 21 AMCO DIAGNOSTICS Abids 0 12 5 3 1 21 22 AMRUTHA DIABETIC CENTRE Somajiguda 5 17 15 5 2 39 23 AMRUTHA HOSPITAL - Parsigutta, SecunderabadParsigutta 8 5 10 5 2 22 24 AMULYA DIAGNOSTICS SERVICES Sanathnagar 0 5 10 3 1 19 25 ANALYZE DIAGNOSTICS LTD. -

December-2017
GJ Multiclave (I) Pvt. Ltd Hyderabad Bio-Medical Waste Collection / Treatment / Disposal report for the Month of December 2017 Quantity in Kgs White Sl. No. Name of the HCE Location Beds Yellow Red Blue (PPC) Total 1 A-Z DIAGNOSTIC & LAB CENTRE Malakpet 0 13 3 12 3 31 2 A.P SUPER SPECIALITY DENTAL HOSPITAL Masab Tank 0 12 6 2 2 22 3 A.P.REDCROSS BLOOD BANK Nallakunta 0 37 28 4 4 73 4 AAROHI BLOOD BANK Rd. No.1 Banjara Hills 0 17 8 4 1 30 5 ACCENTURE SERVICES PVT LTD. ( SEZ ) Nanakramguda 0 6 1 1 1 9 6 ACCESS DENTAL HOSPITAL Alwal 0 5 2 3 1 11 7 ACHARYA TULSI DIAGNOSTICS CENTRE Boinpally 0 2 2 2 2 8 8 ADAM POLY CLINIC & DENTAL CARE Tolichowki 0 2 3 2 2 9 9 ADHI - INDIA - HYDERABAD Rd.No.36, Jubilee Hills 0 2 3 2 2 9 10 ADITYA DIAGNOSTICS, ERRAGADA Erragadda 0 9 2 3 1 15 11 ADVANCE RETINA CARE Panjagutta 0 40 14 4 3 61 12 ADVANCES HAIR STUDIO PVT LTD Banjara Hills 0 10 2 2 1 15 13 AKBAR EYE HOSPITAL Mehdipatnam 0 110 5 2 5 122 14 AKSHARA MEDICAL & RESEARCH CENTRE (Ankura)Begumpet 0 81 12 8 2 103 15 AL-NOOR MATERNITY&GEN HOSPITAL Tolichowki 10 45 6 2 3 56 16 ALL CREATURES ANIMAL CLINIC East Marredpally 0 2 1 2 2 7 17 ALLWIN HOSPITAL Khairthabad 8 38 10 5 6 59 18 ALPHA HOSPITAL Moghalpura 10 33 5 2 3 43 19 AMAR IMMUNODIAGNOSTICS PVT LTD Rd.No.18, Jubilee Hills 0 5 2 1 3 11 20 AMBROSIA CLINIC Rd.No.12, Banjara Hills 0 27 4 2 4 37 21 AMCO DIAGNOSTICS Abids 0 6 2 1 1 10 22 AMRUTHA DIABETIC CENTRE Somajiguda 5 24 5 3 2 34 23 AMRUTHA HOSPITAL - Parsigutta, Secunderabad Parsigutta 8 4 3 2 2 11 24 AMULYA DIAGNOSTICS SERVICES Sanathnagar 0 4 2 2 1 9 25 ANALYZE DIAGNOSTICS LTD. -

Feb 2 5 1987
An Urban Design Study in The Walled City of Hyderabad by Arjun Kamal Mangaldas SUBMITTED TO THE DEPARTMENT OF ARCHITECTURE IN PARTIAL FULFILLMENT OF THE REQUIREMENTS OF THE DEGREE MASTER OF ARCHITECTURE AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 1987 Arjun Kamal Mangaldas 1987 The author hereby grants to M.I.T. permission to reproduce and to distribute publicly copies of this thesis document in whole or in part. Signature of the author Arjun Kamal Mangaldas Department of Architecture December 18, 1986 I N Certified by William Yym n Porter Professor of Architecture and lanning Thesis Supervisor 'A .10 Accepted by -N udy D ytd Miftchell Chairman Departmen al Committee on Graduate Students MaSSCoETSmmSittee MA SSACHUSET TS INSITUTE OF TECHNOLOGY FEB 2 5 1987 UBRARIE5 Room 14-0551 77 Massachusetts Avenue Cambridge, MA 02139 Ph: 617.253.2800 MITLibraries Email: [email protected] Document Services http://Iibraries.mit.edu/docs DISCLAIMER NOTICE The accompanying media item for this thesis is available in the MIT Libraries or Institute Archives. Thank you. An Urban Design Study in the Walled City of Hyderabad by Arjun Kamal Mangaldas Submitted to the Department of Architecture on December 18, 1986 in partial fulfillment of the requirements for the Degree of Master of Architecture ABSTRACT The purpose of this study is to approach the Islamic city in India from a viewpoint which recognizes that the distinctions between cultural sections in a city are not only social and spatial but also physical, being manifest in the built environment. In this sense Hyderabad can be seen as a cross-cultural laboratory for investigating how two or more cultures (Muslim, Hindu, and a distinctly Western/colonial) operated in the same geographical environment but at different levels of social, economic, and physical development under conditions of monarchic rule.