Crystal Field Theory Chapter Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHEM 250 (4 Credits): Reactions of Nucleophiles and Electrophiles (Reactivity 1)
CHEM 250 (4 credits): Reactions of Nucleophiles and Electrophiles (Reactivity 1): Description: This course investigates fundamental carbonyl reactivity (addition and substitution) in understanding organic, inorganic and biochemical processes. Some emphasis is placed on enthalpy, entropy and free energy as a basis of understanding reactivity. An understanding of chemical reactivity is based on principles of Lewis acidity and basicity. The formation, stability and reactivity of coordination complexes are included. Together, these topics lead to an understanding of biochemical pathways such as glycolysis. Enzyme regulation and inhibition is discussed in the context of thermodynamics and mechanisms. Various applications of modern multi-disciplinary research will be explored. Prerequisite: CHEM 125. Course Goals and Objectives: 1. Students will gain a qualitative understanding of thermodynamics. A. Students will develop a qualitative understanding of enthalpy and entropy. B. Students will predict the sign of an entropy change for chemical or physical processes. C. Students will understand entropy effects such as the chelate effect on chemical reactions. D. Students will use bond enthalpies or pKas to determine the enthalpy change for a reaction. E. Students will draw or interpret a reaction progress diagram. F. Students will apply the Principle of Microscopic Reversibility to understand reaction mechanisms. G. Students will use Gibbs free energy to relate enthalpy and entropy changes. H. Students will develop a qualitative understanding of progress toward equilibrium under physiological conditions (the difference between G and Go.) I. Students will understand coupled reactions and how this can be used to drive a non- spontaneous reaction toward products. J. Students will apply LeChatelier’s Principle to affect the position of anequilibrium by adding or removing reactants or products. -

An Earlier Collection of These Notes in One PDF File
UNIVERSITY OF THE WEST INDIES MONA, JAMAICA Chemistry of the First Row Transition Metal Complexes C21J Inorganic Chemistry http://wwwchem.uwimona.edu.jm/courses/index.html Prof. R. J. Lancashire September 2005 Chemistry of the First Row Transition Metal Complexes. C21J Inorganic Chemistry 24 Lectures 2005/2006 1. Review of Crystal Field Theory. Crystal Field Stabilisation Energies: origin and effects on structures and thermodynamic properties. Introduction to Absorption Spectroscopy and Magnetism. The d1 case. Ligand Field Theory and evidence for the interaction of ligand orbitals with metal orbitals. 2. Spectroscopic properties of first row transition metal complexes. a) Electronic states of partly filled quantum levels. l, ml and s quantum numbers. Selection rules for electronic transitions. b) Splitting of the free ion energy levels in Octahedral and Tetrahedral complexes. Orgel and Tanabe-Sugano diagrams. c) Spectra of aquated metal ions. Factors affecting positions, intensities and shapes of absorption bands. 3. Magnetic Susceptibilities of first row transition metal complexes. a) Effect of orbital contributions arising from ground and excited states. b) Deviation from the spin-only approximation. c) Experimental determination of magnetic moments. Interpretation of data. 4. General properties (physical and chemical) of the 3d transition metals as a consequence of their electronic configuration. Periodic trends in stabilities of common oxidation states. Contrast between first-row elements and their heavier congeners. 5. A survey of the chemistry of some of the elements Ti....Cu, which will include the following topics: a) Occurrence, extraction, biological significance, reactions and uses b) Redox reactions, effects of pH on the simple aqua ions c) Simple oxides, halides and other simple binary compounds. -

Crystal Field Theory (CFT)
Crystal Field Theory (CFT) The bonding of transition metal complexes can be explained by two approaches: crystal field theory and molecular orbital theory. Molecular orbital theory takes a covalent approach, and considers the overlap of d-orbitals with orbitals on the ligands to form molecular orbitals; this is not covered on this site. Crystal field theory takes the ionic approach and considers the ligands as point charges around a central metal positive ion, ignoring any covalent interactions. The negative charge on the ligands is repelled by electrons in the d-orbitals of the metal. The orientation of the d orbitals with respect to the ligands around the central metal ion is important, and can be used to explain why the five d-orbitals are not degenerate (= at the same energy). Whether the d orbitals point along or in between the cartesian axes determines how the orbitals are split into groups of different energies. Why is it required? The valence bond approach could not explain the Electronic spectra, Magnetic moments, Reaction mechanisms of the complexes. Assumptions of CFT: 1. The central Metal cation is surrounded by ligand which contain one or more lone pair of electrons. 2. The ionic ligand (F-, Cl- etc.) are regarded as point charges and neutral molecules (H2O, NH3 etc.) as point dipoles. 3. The electrons of ligand does not enter metal orbital. Thus there is no orbital overlap takes place. 4. The bonding between metal and ligand is purely electrostatic i.e. only ionic interaction. The approach taken uses classical potential energy equations that take into account the attractive and repulsive interactions between charged particles (that is, Coulomb's Law interactions). -

Nobel Lecture, 8 December 1981 by ROALD HOFFMANN Department of Chemistry, Cornell University, Ithaca, N.Y
BUILDING BRIDGES BETWEEN INORGANIC AND ORGANIC CHEMISTRY Nobel lecture, 8 December 1981 by ROALD HOFFMANN Department of Chemistry, Cornell University, Ithaca, N.Y. 14853 R. B. Woodward, a supreme patterner of chaos, was one of my teachers. I dedicate this lecture to him, for it is our collaboration on orbital symmetry conservation, the electronic factors which govern the course of chemical reac- tions, which is recognized by half of the 1981 Nobel Prize in Chemistry. From Woodward I learned much: the significance of the experimental stimulus to theory, the craft of constructing explanations, the importance of aesthetics in science. I will try to show you how these characteristics of chemical theory may be applied to the construction of conceptual bridges between inorganic and organic chemistry. FRAGMENTS Chains, rings, substituents - those are the building blocks of the marvelous edifice of modern organic chemistry. Any hydrocarbon may be constructed on paper from methyl groups, CH 3, methylenes, CH 2, methynes, CH, and carbon atoms, C. By substitution and the introduction of heteroatoms all of the skeletons and functional groupings imaginable, from ethane to tetrodotoxin, may be obtained. The last thirty years have witnessed a remarkable renaissance of inorganic chemistry, and the particular flowering of the field of transition metal organo- metallic chemistry. Scheme 1 shows a selection of some of the simpler creations of the laboratory in this rich and ever-growing field. Structures l-3 illustrate at a glance one remarkable feature of transition metal fragments. Here are three iron tricarbonyl complexes of organic moie- ties - cyclobutadiene, trimethylenemethane, an enol, hydroxybutadiene - which on their own would have little kinetic or thermodynamic stability. -

Copyrighted Material
1 INTRODUCTION 1.1 WHY STUDY ORGANOMETALLIC CHEMISTRY? Organometallic chemists try to understand how organic molecules or groups interact with compounds of the inorganic elements, chiefl y metals. These elements can be divided into the main group, consisting of the s and p blocks of the periodic table, and the transition elements of the d and f blocks. Main-group organometallics, such as n -BuLi and PhB(OH)2 , have proved so useful for organic synthesis that their leading characteristics are usually extensively covered in organic chemistry courses. Here, we look instead at the transition metals because their chemistry involves the intervention of d and f orbitals that bring into play reaction pathways not readily accessible elsewhere in the periodic table. While main-group organometallics are typically stoichiometric reagents, many of their transition metal analogs are most effective when they act as catalysts. Indeed, the expanding range of applications of catalysis is a COPYRIGHTEDmajor reason for the continued MATERIAL rising interest in organo- metallics. As late as 1975, the majority of organic syntheses had no recourse to transition metals at any stage; in contrast, they now very often appear, almost always as catalysts. Catalysis is also a central prin- ciple of Green Chemistry 1 because it helps avoid the waste formation, The Organometallic Chemistry of the Transition Metals, Sixth Edition. Robert H. Crabtree. © 2014 John Wiley & Sons, Inc. Published 2014 by John Wiley & Sons, Inc. 1 2 INTRODUCTION for example, of Mg salts from Grignard reactions, that tends to accom- pany the use of stoichiometric reagents. The fi eld thus occupies the borderland between organic and inorganic chemistry. -

Werner-Type Complexes of Uranium(III) and (IV) Judith Riedhammer, J
pubs.acs.org/IC Article Werner-Type Complexes of Uranium(III) and (IV) Judith Riedhammer, J. Rolando Aguilar-Calderon,́ Matthias Miehlich, Dominik P. Halter, Dominik Munz, Frank W. Heinemann, Skye Fortier, Karsten Meyer,* and Daniel J. Mindiola* Cite This: Inorg. Chem. 2020, 59, 2443−2449 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: Transmetalation of the β-diketiminate salt [M]- Me Ph + Me Ph− − [ nacnac ](M =NaorK; nacnac = {PhNC(CH3)}2CH ) with UI3(THF)4 resulted in the formation of the homoleptic, Me Ph octahedral complex [U( nacnac )3](1). Green colored 1 was fully characterized by a solid-state X-ray diffraction analysis and a combination of UV/vis/NIR, NMR, and EPR spectroscopic studies as well as solid-state SQUID magnetization studies and density functional theory calculations. Electrochemical studies of 1 revealed this species to possess two anodic waves for the U(III/IV) and U(IV/V) redox couples, with the former being chemically accessible. Using mild oxidants, such as [CoCp2][PF6]or [FeCp ][Al{OC(CF ) } ], yields the discrete salts [1][A] (A = − 2 3 −3 4 PF6 , Al{OC(CF3)3}4 ), whereas the anion exchange of [1][PF6] with NaBPh4 yields [1][BPh4]. Me Dipp μ 12 ■ INTRODUCTION UCl3(THF)] and [{( nacnac )UCl}2( 2-Cl)3]Cl. In Me Dipp− ’ some cases, disubstitution of nacnac generates the Alfred Werner s disapproval of the complex ion-chain theory Me Dipp η3 Me Dipp 13 proposed by Jørgensen and Blomstrand,1 via the introduction rearranged species [( nacnac )( - nacnac )UI], of coordination complexes and the concept of Nebenvalenz,1,2 where one ligand has changed hapticity, most likely due to fi steric constraints. -

M.Sc. (Chemistry) M
Regulations 2019 Curriculum and Syllabi (Amendments updated upto June 2020) M.Sc. (Chemistry) M. Sc. Chemistry Regulations 2019 REGULATIONS 2019 CURRICULUM AND SYLLABI (Amendments updated upto June 2020) M.Sc. CHEMISTRY B.S. Abdur Rahman Crescent Institute of Science and Technology 1 M. Sc. Chemistry Regulations 2019 B.S. Abdur Rahman Crescent Institute of Science and Technology 2 M. Sc. Chemistry Regulations 2019 VISION AND MISSION OF THE INSTITUTION VISION B.S.Abdur Rahman Crescent Institute of Science and Technology aspires to be a leader in Education, Training and Research in multidisciplinary areas of importance and to play a vital role in the Socio-Economic progress of the Country in a sustainable manner. MISSION To blossom into an internationally renowned Institute. To empower the youth through quality and value-based education. To promote professional leadership and entrepreneurship. To achieve excellence in all its endeavors to face global challenges. To provide excellent teaching and research ambience. To network with global Institutions of Excellence, Business, Industry and Research Organizations. To contribute to the knowledge base through Scientific enquiry, Applied Research and Innovation. B.S. Abdur Rahman Crescent Institute of Science and Technology 3 M. Sc. Chemistry Regulations 2019 B.S. Abdur Rahman Crescent Institute of Science and Technology 4 M. Sc. Chemistry Regulations 2019 DEPARTMENT OF CHEMISTRY VISION AND MISSION VISION To blossom as a department with excellence in the field of Chemical Sciences through academic and research programmes in cutting-edge areas. MISSION To provide knowledge and skill in Chemical Sciences through post graduate and doctoral programmes. To undertake research in emerging areas of Chemical Sciences and transform the findings for the benefit of the society. -

Limitation of Crystal Field Theory the Main Drawback of the Crystal Field Theory Is That It Does Not Consider the Covalent Character in Metal-Ligand Bonding at All
CHAPTER 7 Metal-Ligand Bonding: Limitation of Crystal Field Theory The main drawback of the crystal field theory is that it does not consider the covalent character in metal-ligand bonding at all. It treats the metal-ligand interaction in a purely electrostatic framework which is pretty far from reality. All the effects which originate from covalence cannot be explained by this theory. Therefore, the main limitations of crystal field theory can be concluded only after knowing the causes and magnitude of the covalence in the metal-ligand bonds. Evidences for the Covalent Character in Metal–Ligand Bond The crystal field theory considers the metal center as well as surrounding ligands as point charges and assumes that the interaction between them is 100% ionic. However, quite strong experimental evidences have proved that there is some covalent character too which cannot be ignored. Some of those experimental evidences are as follows: 1. The nephelauxetic effect: The electrons present in the partially filled d-orbitals of the metal center repel each other to produce a number of energy levels. The placement of these levels on the energy scale depends upon the arrangement of filled electrons. The energy of these levels can be given in terms of “Racah parameters” B and C (a measure of interelectronic repulsion). The energy difference between same multiplicity states is expressed in B and Dq while between different multiplicity states is given in term of B, Dq and C. It has been observed that the complexation of metal center always results in a decrease in interelectronic repulsion parameters which in turn also advocates a decrease in the repulsion between d-electron density. -

Organometallic Chemistry BASIC PRINCIPLES, APPLICATIONS, and a FEW CASE STUDIES
Safety Moment TYLER LAB GROUP MEETING 1 Safety Moment TYLER LAB GROUP MEETING 2 Metal Hydrides: Benchtop vs. Box Hydride = :H- Hydrides are powerful Lewis bases and reducing agent ◦ Exothermically form H2 (this should scare you) ◦ Heating leads to faster reactivity ◦ H evolution leads to rapid increase in pressure2 ◦ Uncontrolled reactions easily cause runaway exotherm, class D fire, explosion, and death/unemployment LiAlH is the #1 chemical cause of fatality in chemical4 industry 3 Metal Hydrides: “I want to commit the murder I was imprisoned for†.” LiAlH4 ◦ Insanely irritating (serious safety hazard) ◦ Extremely moisture sensitive (don’t leave out for >2 minutes) ◦ Ethereal mixtures are pyrophoric! DiBuAl-H ◦ Pyrophoric – it will explode upon exposure to oxygen NaEt3BH ◦ Pyrophoric in solution LiH and NaH ◦ Can be handled on the benchtop (not >2 minutes) ◦ Parrafin oil dispersions much safer KH ◦ Pyrophoric if not in a dispersion ◦ Handle with extreme care! † Sirius Black, Harry Potter and the Prisoner of Azkaban 4 Metal Hydrides: “I want to commit the murder I was imprisoned for†.” CaH2 ◦ Very safe to handle on the benchtop Pt-H, Pd-H, Ni-H ◦ All very pyrophoric NaBH4 ◦ Very safe in general Other hydrides ◦ Treat as pyrophoric ◦ Transition metal hydrides vary in hydridic strength ◦ General rule of thumb: if it does hydrogenations, it is probably pyrophoric ◦ If they’re in organics of any kind, they are probably pyrophoric † Sirius Black, Harry Potter and the Prisoner of Azkaban 5 Organometallic Chemistry BASIC PRINCIPLES, APPLICATIONS, -

Ligand Field Theory Continued Chapter 10
Coordination Chemistry II: Ligand Field Theory Continued Chapter 10 Wednesday, November 25, 2015 Adding Metal Electrons * t1u Metal ions typically have some valence electrons that can be accommodated in the metal d orbitals * a1g • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. 1 3+ 4+ 4+ 5+ 5+ * • d ions – Ti , V , Ta , Cr , Mo , etc. eg • d2 ions – V3+, Ta3+, Cr4+, Mo4+, etc. • d3 ions – V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. t2g • d4-d7 – hold on • d8 ions – Co1+, Ni2+, Cu3+, etc. t1u • d9 ions – Ni1+, Cu2+, etc. a1g 10 1+ 2+ eg • d ions – Cu , Zn , etc. High Spin and Low Spin Complexes * * t1u The situation is a little more t1u complicated for d4-d7 metals: * * a1g a1g •d4 ions – Cr2+, Mo2+, Mn3+, Fe4+, Ru4+, etc. * * eg eg •d5 ions – Mn2+, Re2+, Fe3+, Ru3+, etc. LOW SPIN •d6 ions – Fe2+, Ru2+, Co3+, Rh3+, Pt4+, etc. t2g t2g HIGH SPIN •d7 ions – Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc. t t 1u For d4-d7 electron counts: 1u a1g a1g • when ∆o > Πtotal ➙ low spin eg eg • when ∆o < Πtotal ➙ high spin High Spin and Low Spin n+ Electron configurations for octahedral complexes, e.g. [M(H2O)6] . Only the d4 through d7 cases can be either high-spin or low spin. Δ< Π Δ> Π Weak-field ligands: Strong-field ligands: - Small Δ, High spin complexes - Large Δ, Low spin complexes Electron Pairing Energy The total electron pairing energy, Πtotal, has two components, Πc and Πe • Πc is a destabilizing energy for the Coulombic repulsion associated with putting two electrons into the same orbital • Πe is a stabilizing energy for electron exchange associated with -

Organometallics Study Meeting H.Mitsunuma 1
04/21/2011 Organometallics Study Meeting H.Mitsunuma 1. Crystal field theory (CFT) and ligand field theory (LFT) CFT: interaction between positively charged metal cation and negative charge on the non-bonding electrons of the ligand LFT: molecular orbital theory (back donation...etc) Octahedral (figure 9-1a) d-electrons closer to the ligands will have a higher energy than those further away which results in the d-orbitals splitting in energy. ligand field splitting parameter ( 0): energy between eg orbital and t2g orbital 1) high oxidation state 2) 3d<4d<5d 3) spectrochemical series I-< Br-< S2-< SCN-< Cl-< N -,F-< (H N) CO, OH-< ox, O2-< H O< NCS- <C H N, NH < H NCH CH NH < bpy, phen< NO - - - - 3 2 2 2 5 5 3 2 2 2 2 2 < CH3 ,C6H5 < CN <CO cf) pairing energy: energy cost of placing an electron into an already singly occupied orbital Low spin: If 0 is large, then the lower energy orbitals(t2g) are completely filled before population of the higher orbitals(eg) High spin: If 0 is small enough then it is easier to put electrons into the higher energy orbitals than it is to put two into the same low-energy orbital, because of the repulsion resulting from matching two electrons in the same orbital 3 n ex) (t2g) (eg) (n= 1,2) Tetrahedral (figure 9-1b), Square planar (figure 9-1c) LFT (figure 9-3, 9-4) - - Cl , Br : lower 0 (figure 9-4 a) CO: higher 0 (figure 9-4 b) 2. Ligand metal complex hapticity formal chargeelectron donation metal complex hapticity formal chargeelectron donation MR alkyl 1 -1 2 6-arene 6 0 6 MH hydride 1 -1 2 M MX H 1 -1 2 halogen 1 -1 2 M M -hydride M OR alkoxide 1 -1 2 X 1 -1 4 M M -halogen O R acyl 1 -1 2 O 1 -1 4 M R M M -alkoxide O 1-alkenyl 1 -1 2 C -carbonyl 1 0 2 M M M R2 C -alkylidene 1 -2 4 1-allyl 1 -1 2 M M M O C 3-carbonyl 1 0 2 M R acetylide 1 -1 2 MMM R R C 3-alkylidine 1 -3 6 M carbene 1 0 2 MMM R R M carbene 1 -2 4 R M carbyne 1 -3 6 M CO carbonyl 1 0 2 M 2-alkene 2 0 2 M 2-alkyne 2 0 2 M 3-allyl 3 -1 4 M 4-diene 4 0 4 5 -cyclo 5 -1 6 M pentadienyl 1 3. -
High Spin Or Low Spin?
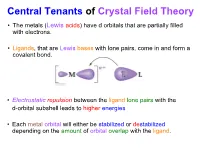
Central Tenants of Crystal Field Theory • The metals (Lewis acids) have d orbitals that are partially filled with electrons. • Ligands, that are Lewis bases with lone pairs, come in and form a covalent bond. • Electrostatic repulsion between the ligand lone pairs with the d-orbital subshell leads to higher energies • Each metal orbital will either be stabilized or destabilized depending on the amount of orbital overlap with the ligand. CFT (Octahedron) (dz2, dx2-y2) Orbitals point directly at ligands eg stronger repulsion dx2y2 E dz2 higher energy (dxy, dyz, dxz) Orbitals point between ligands t2g dxy dyz dxz weaker repulsion lower energy Energy Levels of d-Orbitals in an Octahedron • crystal field splitting energy = • The size of is determined by; metal (oxidation state; row of metal, for 5d > 4d >> 3d) ligand (spectrochemical series) Spectrochemical Series eg eg t2g t2g 3+ [Cr(H2O)6] Greater I < Br < Cl < F < OH < H2O < NH3 < en < NO2 < CN < CO Small Δ Large Δ Weak Field Strong Field Weak M-L interactions Strong M-L interactions How Do the Electrons Go In? • Hund’s rule: one electron each in lowest energy orbitals first • what happens from d4 to d7? d6 Crystal Field Spin Pairing Splitting vs. Δ P High Spin vs. Low Spin Co3+ (d6) High Spin Low Spin Δ < P Δ > P High Spin vs. Low Spin Configurations High Spin vs. Low Spin Configurations high spin low spin d4 3d metal 3d metal weak field strong field ligand d5 ligand 4d & 5d 6 d always d7 What About Other Geometries? linear (CN = 2) z y x tetrahedral (CN = 4) z y x square planar (CN = 4) z how do the electrons on the d orbitals y interact with the ligands in these cases? x Tetrahedral vs.