Single-Cell Transcriptomic and Functional Characterization Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variation in Protein Coding Genes Identifies Information
bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 1 1 2 3 4 5 Variation in protein coding genes identifies information flow as a contributor to 6 animal complexity 7 8 Jack Dean, Daniela Lopes Cardoso and Colin Sharpe* 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Institute of Biological and Biomedical Sciences 25 School of Biological Science 26 University of Portsmouth, 27 Portsmouth, UK 28 PO16 7YH 29 30 * Author for correspondence 31 [email protected] 32 33 Orcid numbers: 34 DLC: 0000-0003-2683-1745 35 CS: 0000-0002-5022-0840 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Abstract bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 2 1 Across the metazoans there is a trend towards greater organismal complexity. How 2 complexity is generated, however, is uncertain. Since C.elegans and humans have 3 approximately the same number of genes, the explanation will depend on how genes are 4 used, rather than their absolute number. -

Wild-Derived Allele of Tmem173 Potentiates an Alternate Signaling Response to Cytosolic DNA
Wild-derived allele of Tmem173 potentiates an alternate signaling response to cytosolic DNA. A dissertation submitted by Guy Surpris In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Immunology TUFTS UNIVERSITY Sackler School of Graduate Biomedical Sciences May 2016 Adviser: Alexander Poltorak ABSTRACT The cellular recognition of cytosolic DNA is critical for maintaining homeostasis and to signal warnings to prevent the spread of pathogens such as HSV1 or Listeria. Inborn mutations in the human population determine the susceptibility or ability to clear infection. Mouse models of infectious disease are an invaluable resource for the study of these mechanisms of disease progression. However, classical laboratory mouse strains do not always recapitulate the diversity in immune responses found in the human population. Wild derived mice are an excellent source of genomic and phenotype diversity in the lab. Herein, we report and characterize phenotype variations in the wild-derived mouse strain MOLF/Ei and classical lab mouse strain in interferon stimulated gene induction to cytosolic DNA species. Using forward genetic analysis, we identified multiple loci that confer attenuated IFNβ production in MOLF/Ei macrophages to pathogen derived cytosolic di-nucleotides. Fine mapping of a major locus of linkage revealed a novel polymorphic allele of Tmem173 (STING). The MOLF allele of Tmem173 produces a protein with multiple amino acid changes, and an internal 6 amino acid deletion. Most of these amino acid changes are confined to the understudied N-terminus. These polymorphisms in MOLF STING altogether confer a lack of induction of the IFNβ promoter in an overexpression assay that seems to be attributed to the most N-terminal proximal mutations. -

Evaluating the Repair of DNA Derived from Formalin-Fixed Paraffin
Laboratory Investigation (2013) 93, 701–710 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Evaluating the repair of DNA derived from formalin-fixed paraffin-embedded tissues prior to genomic profiling by SNP–CGH analysis Abdel Nasser Hosein1,*, Sarah Song2,3,*, Amy E McCart Reed3, Janani Jayanthan3, Lynne E Reid3, Jamie R Kutasovic3, Margaret C Cummings3,4, Nic Waddell2, Sunil R Lakhani3,4,5, Georgia Chenevix-Trench1 and Peter T Simpson3 Pathology archives contain vast resources of clinical material in the form of formalin-fixed paraffin-embedded (FFPE) tissue samples. Owing to the methods of tissue fixation and storage, the integrity of DNA and RNA available from FFPE tissue is compromized, which means obtaining informative data regarding epigenetic, genomic, and expression altera- tions can be challenging. Here, we have investigated the utility of repairing damaged DNA derived from FFPE tumors prior to single-nucleotide polymorphism (SNP) arrays for whole-genome DNA copy number analysis. DNA was extracted from FFPE samples spanning five decades, involving tumor material obtained from surgical specimens and postmortems. Various aspects of the protocol were assessed, including the method of DNA extraction, the role of Quality Control quantitative PCR (qPCR) in predicting sample success, and the effect of DNA restoration on assay performance, data quality, and the prediction of copy number aberrations (CNAs). DNA that had undergone the repair process yielded higher SNP call rates, reduced log R ratio variance, and improved calling of CNAs compared with matched FFPE DNA not subjected to repair. Reproducible mapping of genomic break points and detection of focal CNAs representing high- level gains and homozygous deletions (HD) were possible, even on autopsy material obtained in 1974. -

Spatial Localization of Genes Determined by Intranu- Clear DNA Fragmentation with the Fusion Proteins Lamin KRED and Histone
Int. J. Med. Sci. 2013, Vol. 10 1136 Ivyspring International Publisher International Journal of Medical Sciences 2013; 10(9):1136-1148. doi: 10.7150/ijms.6121 Research Paper Spatial Localization of Genes Determined by Intranu- clear DNA Fragmentation with the Fusion Proteins Lamin KRED and Histone KRED und Visible Light Waldemar Waldeck1, Gabriele Mueller1, Karl-Heinz Glatting3, Agnes Hotz-Wagenblatt3, Nicolle Diessl4, Sasithorn Chotewutmonti4, Jörg Langowski1, Wolfhard Semmler2, Manfred Wiessler2 and Klaus Braun2 1. German Cancer Research Center, Dept. of Biophysics of Macromolecules, INF 580, D-69120 Heidelberg, Germany; 2. German Cancer Research Center, Dept. of Medical Physics in Radiology, INF 280, D-69120 Heidelberg, Germany; 3. German Cancer Research Center, Genomics Proteomics Core Facility HUSAR Bioinformatics Lab, INF 580, D-69120 Heidelberg, Ger- many; 4. German Cancer Research Center, Genomics and Proteomics Core Facility High Throughput Sequencing, INF 580, D-69120 Heidelberg, Germany. Corresponding author: Dr. Klaus Braun, German Cancer Research Center (DKFZ), Dept. of Medical Physics in Radiology, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. Phone: +49 6221-42 3329 Fax: +49 6221-42 3326 e-mail: [email protected]. © Ivyspring International Publisher. This is an open-access article distributed under the terms of the Creative Commons License (http://creativecommons.org/ licenses/by-nc-nd/3.0/). Reproduction is permitted for personal, noncommercial use, provided that the article is in whole, unmodified, and properly cited. Received: 2013.02.22; Accepted: 2013.06.06; Published: 2013.07.07 Abstract The highly organized DNA architecture inside of the nuclei of cells is accepted in the scientific world. In the human genome about 3 billion nucleotides are organized as chromatin in the cell nucleus. -

C13orf1 (SPRYD7) (NM 001127482) Human Over-Expression Lysate Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for LY426797 C13orf1 (SPRYD7) (NM_001127482) Human Over-expression Lysate Product data: Product Type: Over-expression Lysates Description: Transient overexpression lysate of chromosome 13 open reading frame 1 (C13orf1), transcript variant 2 Species: Human Expression Host: HEK293T Expression cDNA Clone TrueORF Clone RC225140 or AA Sequence: Tag: C-Myc/DDK Detection Antibodies: Clone OTI4C5, Anti-DDK (FLAG) monoclonal antibody (TA50011-100) Accession Number: NM_001127482, NP_001120954 Synonyms: C13orf1; CLLD6 Predicted MW: 17.3 kDa Components: 1 vial of 100 µg gene specific transient over-expression cell lysate in RIPA buffer 1 vial of 100 µg empty vector transfected control cell lysate in RIPA buffer 1 vial of 250ul 2xSDS Sample Buffer (4% SDS, 125mM Tris-HCl pH6.8, 10% Glycerol, 0.002% Bromphenol blue, 100mM DTT) Storage: The lysate is shipped with dry ice. The lysate is shipped with dry ice. Upon receiving, store the sample at -80°C. Also after dilution, the protein sample should be aliquoted and stored at - 80°C for long term storage. Avoid repeated freeze-thaw cycles. Lysate samples can be diluted with 2xSDS Sample Buffer provided. Lysate samples are stable for 12 months from the date of receipt when stored at -80°C. Preparation: HEK293T cells in 10-cm dishes were transiently transfected withM egaTran Transfection Reagent (TT200002) and 5ug TrueORF cDNA plasmid. Transfected cells were cultured for 48hrs before collection. The cells were lysed in modified RIPA buffer (25mM Tris-HCl pH7.6, 150mM NaCl, 1% NP-40, 1mM EDTA, 1xProteinase inhibitor cocktail mix (Sigma), 1mM PMSF and 1mM Na3VO4), and then centrifuged to clarify the lysate. -

Variation in Protein Coding Genes Identifies Information Flow
bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 1 1 2 3 4 5 Variation in protein coding genes identifies information flow as a contributor to 6 animal complexity 7 8 Jack Dean, Daniela Lopes Cardoso and Colin Sharpe* 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Institute of Biological and Biomedical Sciences 25 School of Biological Science 26 University of Portsmouth, 27 Portsmouth, UK 28 PO16 7YH 29 30 * Author for correspondence 31 [email protected] 32 33 Orcid numbers: 34 DLC: 0000-0003-2683-1745 35 CS: 0000-0002-5022-0840 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Abstract bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 2 1 Across the metazoans there is a trend towards greater organismal complexity. How 2 complexity is generated, however, is uncertain. Since C.elegans and humans have 3 approximately the same number of genes, the explanation will depend on how genes are 4 used, rather than their absolute number. -

Agricultural University of Athens
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΤΩΝ ΖΩΩΝ ΤΜΗΜΑ ΕΠΙΣΤΗΜΗΣ ΖΩΙΚΗΣ ΠΑΡΑΓΩΓΗΣ ΕΡΓΑΣΤΗΡΙΟ ΓΕΝΙΚΗΣ ΚΑΙ ΕΙΔΙΚΗΣ ΖΩΟΤΕΧΝΙΑΣ ΔΙΔΑΚΤΟΡΙΚΗ ΔΙΑΤΡΙΒΗ Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων ΕΙΡΗΝΗ Κ. ΤΑΡΣΑΝΗ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ: ΑΝΤΩΝΙΟΣ ΚΟΜΙΝΑΚΗΣ ΑΘΗΝΑ 2020 ΔΙΔΑΚΤΟΡΙΚΗ ΔΙΑΤΡΙΒΗ Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων Genome-wide association analysis and gene network analysis for (re)production traits in commercial broilers ΕΙΡΗΝΗ Κ. ΤΑΡΣΑΝΗ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ: ΑΝΤΩΝΙΟΣ ΚΟΜΙΝΑΚΗΣ Τριμελής Επιτροπή: Aντώνιος Κομινάκης (Αν. Καθ. ΓΠΑ) Ανδρέας Κράνης (Eρευν. B, Παν. Εδιμβούργου) Αριάδνη Χάγερ (Επ. Καθ. ΓΠΑ) Επταμελής εξεταστική επιτροπή: Aντώνιος Κομινάκης (Αν. Καθ. ΓΠΑ) Ανδρέας Κράνης (Eρευν. B, Παν. Εδιμβούργου) Αριάδνη Χάγερ (Επ. Καθ. ΓΠΑ) Πηνελόπη Μπεμπέλη (Καθ. ΓΠΑ) Δημήτριος Βλαχάκης (Επ. Καθ. ΓΠΑ) Ευάγγελος Ζωίδης (Επ.Καθ. ΓΠΑ) Γεώργιος Θεοδώρου (Επ.Καθ. ΓΠΑ) 2 Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων Περίληψη Σκοπός της παρούσας διδακτορικής διατριβής ήταν ο εντοπισμός γενετικών δεικτών και υποψηφίων γονιδίων που εμπλέκονται στο γενετικό έλεγχο δύο τυπικών πολυγονιδιακών ιδιοτήτων σε κρεοπαραγωγικά ορνίθια. Μία ιδιότητα σχετίζεται με την ανάπτυξη (σωματικό βάρος στις 35 ημέρες, ΣΒ) και η άλλη με την αναπαραγωγική -
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
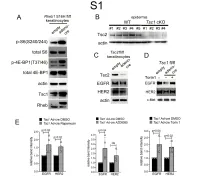
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test). -

Discovery and Fine-Mapping of Adiposity Loci Using High Density
Henry Ford Health System Henry Ford Health System Scholarly Commons Center for Health Policy and Health Services Center for Health Policy and Health Services Research Articles Research 4-1-2017 Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium Maggie CY Ng Mariaelisa Graff Yingchang Lu FAnneollow E. this Justice and additional works at: https://scholarlycommons.henryford.com/chphsr_articles Poorva Mudgal Recommended Citation Ng MC, Graff M, Lu Y, Justice AE, Mudgal P, Liu C, Young K, Yanek LR, Feitosa MF, Wojczynski MK, Rand K, BrSeeody next JA, page Cade for BE, additional Dimitrov L,authors Duan Q, Guo X, Lange LA, Nalls M, Okut H, Tajuddin SM, Tayo BO, Vedantam S, Bradfield JP, Chen G, Chen W, Chesi A, Irvin MR, Padhukasahasram B, Smith JA, Zheng W, Allison MA, Ambrosone CB, Bandera E, Bartz T, Berndt S, Bernstein L, Blot W, Bottinger E, Carpten J, Chanock S, Chen Y, Conti D, Cooper R, Fornage M, Freedman B, Garcia M, Goodman P, Hsu Y, Hu J, Huff C, Ingles S, John E, Kittles R, Klein E, Li J, McKnight B, Nayak U, Nemesure B, Ogunniyi A, Olshan A, Press M, Rohde R, Rybicki BA, Salako B, Sanderson M, Shao Y, Siscovick D, Stanford J, Stevens V, Stram A, Strom S, Vaidya D, Witte J, Yao J, Zhu X, Ziegler R, Zonderman A, Adeyemo A, Ambs S, Cushman M, Faul J, Hakonarson H, Levin AM, Nathanson K, Ware E, Weir D, Zhao W, Zhi D, Arnett D, Grant S, Kardia S, Oloapde O, Rao D, Rotimi C, Sale M, Williams KL, Zemel B, Becker D, Borecki I, Evans M, Harris T, Hirschhorn J, Li Y, Patel S, Psaty B, Rotter J, Wilson J, Bowden D, Cupples L, Haiman C, Loos R, North K. -

Regular Article
From bloodjournal.hematologylibrary.org at MIT LIBRARIES on January 28, 2014. For personal use only. Regular Article RED CELLS, IRON, AND ERYTHROPOIESIS Global discovery of erythroid long noncoding RNAs reveals novel regulators of red cell maturation Juan R. Alvarez-Dominguez,1,2 Wenqian Hu,1 Bingbing Yuan,1 Jiahai Shi,1 Staphany S. Park,1,2,3 Austin A. Gromatzky,1,4 Alexander van Oudenaarden,2,5,6,7 and Harvey F. Lodish1,2,4 1Whitehead Institute for Biomedical Research, Cambridge, MA; 2Department of Biology, 3Department of Electrical Engineering and Computer Science, 4Department of Biological Engineering, and 5Department of Physics, Massachusetts Institute of Technology, Cambridge, MA; 6Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences, Utrecht, The Netherlands; and 7University Medical Center Utrecht, Utrecht, The Netherlands Key Points Erythropoiesis is regulated at multiple levels to ensure the proper generation of mature red cells under multiple physiological conditions. To probe the contribution of long noncoding • Global lncRNA discovery RNAs (lncRNAs) to this process, we examined >1 billion RNA-seq reads of polyadenylated reveals novel erythroid- and nonpolyadenylated RNA from differentiating mouse fetal liver red blood cells and specific lncRNAs that are identified 655 lncRNA genes including not only intergenic, antisense, and intronic but also dynamically expressed and pseudogene and enhancer loci. More than 100 of these genes are previously unrecognized targeted by GATA1, TAL1, and highly erythroid specific. By integrating genome-wide surveys of chromatin states, and KLF1. transcription factor occupancy, and tissue expression patterns, we identify multiple lncRNAs that are dynamically expressed during erythropoiesis, show epigenetic regula- • Multiple types of lncRNAs tion, and are targeted by key erythroid transcription factors GATA1, TAL1, or KLF1. -

Supplementary Table S1. Protein Identification Parameters Item Value
Supplementary Table S1. Protein identification parameters Item Value Enzyme Trypsin Max Missed Cleavages 2 Carbamidomethyl (C), Fixed modifications TMT 10 plex (N-term), TMT 10 plex (K) Variable modifications Oxidation (M) Peptide Mass Tolerance ± 20 ppm Fragment Mass Tolerance 0.1Da Database uniprot_rat_36079_20170921.fasta Database pattern Decoy Peptide FDR ≤0.01 The protein ratios are calculated as the median of Protein Quantification only unique peptides of the protein Normalizes all peptide ratios by the median protein Experimental Bias ratio. The median protein ratio should be 1 after the normalization. Supplementary Table S2. List of protein quantification and differential analysis Accession Description HF/C t test p value Cytochrome P450 2C70 OS=Rattus norvegicus GN=Cyp2c70 P19225 1.808583525 9.953E-07 PE=2 SV=1 - [CP270_RAT] Carboxylic ester hydrolase OS=Rattus norvegicus G3V9D8 1.583811454 1.61313E-06 GN=LOC108348093 PE=1 SV=1 - [G3V9D8_RAT] Epoxide hydrolase 1 OS=Rattus norvegicus GN=Ephx1 PE=1 P07687 1.732555816 3.21041E-06 SV=1 - [HYEP_RAT] Cholesterol 7-alpha-monooxygenase OS=Rattus norvegicus P18125 1.398497925 4.77469E-06 GN=Cyp7a1 PE=1 SV=1 - [CP7A1_RAT] Methyltransferase-like protein 7B OS=Rattus norvegicus Q562C4 1.462168835 5.07554E-06 GN=Mettl7b PE=1 SV=1 - [MET7B_RAT] Serum paraoxonase/arylesterase 1 OS=Rattus norvegicus P55159 1.451799589 8.48466E-06 GN=Pon1 PE=1 SV=3 - [PON1_RAT] Estradiol 17-beta-dehydrogenase 11 OS=Rattus norvegicus Q6AYS8 1.652588897 1.39328E-05 GN=Hsd17b11 PE=2 SV=1 - [DHB11_RAT] Peroxisomal -

Association of Cyclin-Dependent Kinase Inhibitor 2B Antisense RNA 1 Gene Expression and Rs2383207 Variant with Breast Cancer
Kattan et al. Cell Mol Biol Lett (2021) 26:14 https://doi.org/10.1186/s11658‑021‑00258‑9 Cellular & Molecular Biology Letters RESEARCH Open Access Association of cyclin‑dependent kinase inhibitor 2B antisense RNA 1 gene expression and rs2383207 variant with breast cancer risk and survival Shahad W. Kattan1, Yahya H. Hobani2, Sameerah Shaheen3, Sara H. Mokhtar4, Mohammad H. Hussein5, Eman A. Toraih5,6, Manal S. Fawzy7,8* and Hussein Abdelaziz Abdalla9,10 *Correspondence: [email protected] Abstract 7 Department of Medical Background: The expression signature of deregulated long non-coding RNAs Biochemistry and Molecular Biology, Faculty of Medicine, (lncRNAs) and related genetic variants is implicated in every stage of tumorigenesis, Suez Canal University, Ismailia, progression, and recurrence. This study aimed to explore the association of lncRNA Egypt cyclin-dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B-AS1) gene expression Full list of author information is available at the end of the and the rs2383207A>G intronic variant with breast cancer (BC) risk and prognosis and article to verify the molecular role and networks of this lncRNA in BC by bioinformatics gene analysis. Methods: Serum CDKN2B-AS1 relative expression and rs2383207 genotypes were determined in 214 unrelated women (104 primary BC and 110 controls) using real-time PCR. Sixteen BC studies from The Cancer Genome Atlas (TCGA) including 8925 patients were also retrieved for validation of results. Results: CDKN2B-AS1 serum levels were upregulated in the BC patients relative to controls. A/A genotype carriers were three times more likely to develop BC under homozygous (OR 3.27, 95% CI 1.20–8.88, P 0.044) and recessive (OR 3.17, 95% CI 1.20–8.34, P 0.013)= models.