From Mercury to a Coreless Terrestrial Planet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tregloan-Reed Phd 2014.Pdf
This work is protected by copyright and other intellectual property rights and duplication or sale of all or part is not permitted, except that material may be duplicated by you for research, private study, criticism/review or educational purposes. Electronic or print copies are for your own personal, non- commercial use and shall not be passed to any other individual. No quotation may be published without proper acknowledgement. For any other use, or to quote extensively from the work, permission must be obtained from the copyright holder/s. Starspot properties and photometric parameters of transiting planets and their host stars Jeremy Tregloan-Reed Mphys (Hons) Lancaster University Doctor of Philosophy Department of Astrophysics, University of Keele. October 2014 iii Abstract To begin understanding how the architecture of hot Jupiter planetary systems can be so radically different from that of our own solar system, requires the dynamical evolution of planets to be known. By measuring the sky-projected obliquity λ of a system it is possible to determine the dominant process in the dynamical evolution. If a transiting exoplanet that crosses the disc of its host star passes over a starspot, then the amount of received intensity from the star will change. By modelling the position of the anomaly in the lightcurve it is possible to precisely determine the position of the starspot on the stellar disc. If the position of the starspot can be found at two distinct times using two closely spaced transits, then it is possible to measure λ. Before now there was no definitive model capable of accurately modelling both a planetary transit and a starspot. -

A Possible Explanation of the Secular Increase of the Astronomical Unit 1
T. Miura 1 A possible explanation of the secular increase of the astronomical unit Takaho Miura(a), Hideki Arakida, (b) Masumi Kasai(a) and Syuichi Kuramata(a) (a)Faculty of Science and Technology,Hirosaki University, 3 bunkyo-cyo Hirosaki, Aomori, 036-8561, Japan (b)Education and integrate science academy, Waseda University,1 waseda sinjuku Tokyo, Japan Abstract We give an idea and the order-of-magnitude estimations to explain the recently re- ported secular increase of the Astronomical Unit (AU) by Krasinsky and Brumberg (2004). The idea proposed is analogous to the tidal acceleration in the Earth-Moon system, which is based on the conservation of the total angular momentum and we apply this scenario to the Sun-planets system. Assuming the existence of some tidal interactions that transfer the rotational angular momentum of the Sun and using re- d ported value of the positive secular trend in the astronomical unit, dt 15 4(m/s),the suggested change in the period of rotation of the Sun is about 21(ms/cy) in the case that the orbits of the eight planets have the same "expansionrate."This value is suf- ficiently small, and at present it seems there are no observational data which exclude this possibility. Effects of the change in the Sun's moment of inertia is also investi- gated. It is pointed out that the change in the moment of inertia due to the radiative mass loss by the Sun may be responsible for the secular increase of AU, if the orbital "expansion"is happening only in the inner planets system. -

Open Research Online Oro.Open.Ac.Uk
Open Research Online The Open University’s repository of research publications and other research outputs Extra-Solar Planetary Atmospheres and Interior Structure: Implications for Observational Signatures Thesis How to cite: Bending, Victoria Louise (2016). Extra-Solar Planetary Atmospheres and Interior Structure: Implications for Observational Signatures. PhD thesis The Open University. For guidance on citations see FAQs. c 2016 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.0000ef7b Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Extra-Solar Planetary Atmospheres and Interior Structure: Implications for Observational Signatures Victoria Louise Bending BA (Hons) (Open University) 2004 MPhys (Durham) 2009 This thesis is submitted for the degree of Doctor of Philosophy Department of Physical Sciences The Open University Submitted: October 2015 ProQuest Number: 13834775 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13834775 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. -

Planetary Seismology
This article was originally published in Treatise on Geophysics, Second Edition, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Lognonné P., and Johnson C.L Planetary Seismology. In: Gerald Schubert (editor-in- chief) Treatise on Geophysics, 2nd edition, Vol 10. Oxford: Elsevier; 2015. p. 65-120. Author's personal copy 10.03 Planetary Seismology P Lognonne´, Universite´ Paris Diderot -Sorbonne Paris Cite´, Institut de Physique du Globe de Paris, Paris, France CL Johnson, University of British Columbia, Vancouver, BC, Canada; Planetary Science Institute, Tucson, AZ, USA ã 2015 Elsevier B.V. All rights reserved. 10.03.1 Introduction 65 10.03.2 Lunar Results 70 10.03.2.1 The Apollo PSE Data 70 10.03.2.2 Seismic-Velocity Structure: Crust and Mantle 72 10.03.2.3 Very Deep Interior 77 10.03.2.4 Mineralogical and Thermal Interpretation of Lunar Seismic -

DENSITY of the MANTLE of MARS Kenneth A. Goettel, Dept. of Earth and Planetary Sciences, Mcdonnell Center for the Space Sciences, Washington, University, St
DENSITY OF THE MANTLE OF MARS Kenneth A. Goettel, Dept. of Earth and Planetary Sciences, McDonnell Center for the Space Sciences, Washington, University, St. Louis, MO 63130 The zero pressure density of the Martian mantle is an important constraint on the composition and mineralogy of the mantle. The mean density and moment of inertia factor of Mars constrain the density of the Martian mantle, but do not define it uniquely. The mean density of Mars, 3.933+0.002 g/cm3 (1) is well determined. The estimated moment of inertia factor wFich Mars would have if Mars were in hydrostatic equilibrium, 0.365 (2,3), may still be significantly model dependent. With these constraints, the estimated zero pressure density of the Martian mantle varies as a function of the core composition assumed and as a function of the elastic data chosen as represent- ative of the Martian interior. Recent estimates of the zero pressure density of the Martian mantle are shown in Table 1. The results of Johnston et a1 . (4) were based on a value of 0.377 for the moment of inertia factor of Mars. The results of Johnston and ToksBz (5) and Okal and Anderson (6) were both based on the revised (2,3) value of 0.365 for the moment of inertia factor. The results of (5) and (6) are not completely comparable, since (5) did not give the densities assumed for their core compositions, and (6) did not give compositions corresponding to their core densities. Part of the motivation for the present work was to explore the reasons for the differences between the results of (5) and (6). -

Interior Structure of Terrestrial Planets Tilman Spohn Überblick Überblick Komplexe Systeme Weltraum
Interior Structure of Terrestrial Planets Tilman Spohn Überblick Überblick Komplexe Systeme Weltraum ErosionErosion durchdurch Sonnenwind;Sonnenwind; ImpakteImpakte Biosphäre Hydrosphäre Atmosphäre Kruste Biogeochemiche Wechselwirkungen stabilisieren die VulkanismusVulkanismus AusgasenAusgasen Oberflächentemperatur (Kohlenstoff Zyklus) Mantel Weltraum Magnetosphäre ErosionErosion durchdurch AbschiirmungAbschiirmung Sonnenwind;Sonnenwind; ImpakteImpakte Biosphäre Hydrosphäre Atmosphäre Kruste Subduktion,Subduktion, VolatilrückführungVolatilrückführung undund VulkanismusVulkanismus AusgasenAusgasen verstärkteverstärkte KühlungKühlung Mantel KonvektiveKonvektive KühlungKühlung Kern DynamoDynamo Die Allianz Interior Structure 7 Tilman Spohn, IfP Münster 0.5 ME 0.8 ME 1 ME 2.5 ME 5 ME Interior Structure Interior Structure models aim at determining Mars the masses of major chemical reservoirs the depths to chemical discontinuities and phase transition boundaries the variation with depth of thermodynamic state variables (r, P, T) and transport parameters Folie 9 > Spohn Of Particular Interest… Core radius State of the core Inner core radius Heat flow from the core Transport parameters Folie 10 > Spohn Interior Structure Constraints Mass Moment of inertia factor Gravity field, Topography Rotation parameters Tides Surface rock chemistry/ mineralogy Cosmochemical constraints Laboratory data Magnetism MGS Gravity Field of Mars Future: Seismology! Heat flow Folie 11 > Spohn Doppler Principle 12 T. Spohn, IfP June 24, 2003 Mars Jupiter Ganymede Chemical Components: Gas (H, He), Ice (NH3, CH4, H2O), Rock/Iron 13 Page 14 > Objectives and Accomplishments > Prof. Dr.Tilman Spohn Chemistry Chondritic ratio Fe/Si = 1.71 Mg2SiO4 (olivine) mantle and Fe core 16 Surface Rock 17 SNC Meteorites Samples from Mars? SNC: Shergotty,Nahkla, Chassigny Most of them are young: a few 100 million to 1 billion years Basalts 18 Tilman Spohn, IfP Münster 1st HGF Alliance Week, Berlin,14 May 2008 Dynamic Tidal Response Constraint Tidal deformation is characterized by .. -

Enceladus: Present Internal Structure and Differentiation by Early and Long-Term Radiogenic Heating
Icarus 188 (2007) 345–355 www.elsevier.com/locate/icarus Enceladus: Present internal structure and differentiation by early and long-term radiogenic heating Gerald Schubert a,b,∗, John D. Anderson c,BryanJ.Travisd, Jennifer Palguta a a Department of Earth and Space Sciences, University of California, 595 Charles E. Young Drive East, Los Angeles, CA 90095-1567, USA b Institute of Geophysics and Planetary Physics, University of California, 603 Charles E. Young Drive East, Los Angeles, CA 90095-1567, USA c Global Aerospace Corporation, 711 West Woodbury Road, Suite H, Altadena, CA 91001-5327, USA d Earth & Environmental Sciences Division, EES-2/MS-F665, Los Alamos National Laboratory, Los Alamos, NM 87545, USA Received 30 May 2006; revised 27 November 2006 Available online 8 January 2007 Abstract Pre-Cassini images of Saturn’s small icy moon Enceladus provided the first indication that this satellite has undergone extensive resurfacing and tectonism. Data returned by the Cassini spacecraft have proven Enceladus to be one of the most geologically dynamic bodies in the Solar System. Given that the diameter of Enceladus is only about 500 km, this is a surprising discovery and has made Enceladus an object of much interest. Determining Enceladus’ interior structure is key to understanding its current activity. Here we use the mean density of Enceladus (as determined by the Cassini mission to Saturn), Cassini observations of endogenic activity on Enceladus, and numerical simulations of Enceladus’ thermal evolution to infer that this satellite is most likely a differentiated body with a large rock-metal core of radius about 150 to 170 km surrounded by a liquid water–ice shell. -

Dynamical Measurements of the Interior Structure of Exoplanets
The Astrophysical Journal, 778:100 (11pp), 2013 December 1 doi:10.1088/0004-637X/778/2/100 C 2013. The American Astronomical Society. All rights reserved. Printed in the U.S.A. DYNAMICAL MEASUREMENTS OF THE INTERIOR STRUCTURE OF EXOPLANETS Juliette C. Becker1 and Konstantin Batygin2 1 Cahill Center for Astronomy and Astrophysics, California Institute of Technology, 1200 E. California Blvd., Pasadena, CA 91125, USA; [email protected] 2 Institute for Theory and Computation, Harvard-Smithsonian Center for Astrophysics, 60 Garden St., Cambridge, MA, USA Received 2013 January 3; accepted 2013 September 11; published 2013 November 8 ABSTRACT Giant gaseous planets often reside on orbits in sufficient proximity to their host stars for the planetary quadrupole gravitational field to become non-negligible. In presence of an additional planetary companion, a precise characterization of the system’s orbital state can yield meaningful constraints on the transiting planet’s interior structure. However, such methods can require a very specific type of system. This paper explores the dynamic range of applicability of these methods and shows that interior structure calculations are possible for a wide array of orbital architectures. The HAT-P-13 system is used as a case study, and the implications of perturbations arising from a third distant companion on the feasibility of an interior calculation are discussed. We find that the method discussed here is likely to be useful in studying other planetary systems, allowing the possibility of an expanded survey of the interiors of exoplanets. Key words: planets and satellites: dynamical evolution and stability – planets and satellites: interiors Online-only material: color figures 1. -

Geology of Dwarf Planet Ceres and Meteorite Analogs H
80th Annual Meeting of the Meteoritical Society 2017 (LPI Contrib. No. 1987) 6003.pdf GEOLOGY OF DWARF PLANET CERES AND METEORITE ANALOGS H. Y. McSween1, C. A. Raymond2, T. H. Prettyman3, M. C. De Sanctis4, J. C. Castillo-Rogez2, C. T. Russell5, and the Dawn Science Team, 1Department of Earth & Planetary Sciences, University of Tennessee, Knoxville, TN 37996-1410, [email protected]. 2Jet Propulsion Laboratory, Pasadena, CA 91109, 3Planetary Science Institute, Tucson, AZ 85719, 4Istituto di Astrofisica e Planetologia Spaziali, Istituto Nazionale di Astrofisica, Rome, Italy, 5Department of Earth, Planetary and Space Sciences, University of California, Los Angeles, CA 90095. Introduction: Although no known meteorites are thought to derive from Ceres, CI/CM carbonaceous chondrites are potential analogs for its composition and alteration [1]. These meteorites have primitive chemical compositions, despite having suffered aqueous alteration; this paradox can be explained by closed-system alteration in small parent bodies where low permeability limited fluid transport. In contrast, Ceres, the largest asteroidal body, has experi- enced extensive alteration accompanied by differentiation of silicates and volatiles [2]. The ancient surface of Ceres has a crater density similar to Vesta, but basins >400 km are absent or relaxed. The Dawn spacecraft’s GRaND instrument revealed near-surface ice concentrations in the regolith at high latitudes [3], exposed on the surface locally in a few craters [4]. The VIR instrument indicates a dark surface interpreted as a lag deposit from ice sublimation and composed of ammoniated clays, serpentine, MgCa-carbonates, and a darkening component [5]. Elemental analysis of Fe and H abundances in the non-icy regolith are lower and higher, respective- ly, than for CI/CM chondrites [3]. -
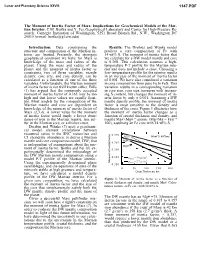
The Moment of Inertia Factor of Mars: Implications for Geochemical Models of the Mar- Tian Interior
Lunar and Planetary Science XXVIII 1147.PDF The Moment of Inertia Factor of Mars: Implications for Geochemical Models of the Mar- tian Interior. C.M. Bertka and Y. Fei, Geophysical Laboratory and Center for High-Pressure Re- search, Carnegie Institution of Washington, 5251 Broad Branch Rd., N.W., Washington DC 20015 (e-mail: [email protected]) Introduction: Data constraining the Results: The Dreibus and Wänke model structure and composition of the Martian in- predicts a core composition of Fe with terior are limited. Presently, the strongest 14 wt% S. The moment of inertia factor that geophysical constraint we have for Mars is we calculate for a DW model mantle and core knowledge of the mass and radius of the is 0.368. This calculation assumes a high- planet. Using the mass and radius of the temperature P-T profile for the Martian inte- planet and the moment of inertia factor as rior and does not include a crust. Choosing a constraints, two of three variables, mantle low-temperature profile for the interior results density, core size, and core density, can be in an increase of the moment of inertia factor calculated as a function of one of the three of 0.001. We have also considered a variation variables. Unfortunately, the Martian moment in core composition from pure Fe to FeS. This of inertia factor is not well known either. Bills variation results in a corresponding variation (1) has argued that the commonly accepted in core size, core size increases with increas- moment of inertia factor of 0.365 may be too ing S content, but changes the moment of in- high and that lower values are equally feasi- ertia factor by only ± 0.001. -

A Precise Carbon and Oxygen Abundance Determination in a Hot Jupiter Atmosphere
A Precise Carbon and Oxygen Abundance Determination in a Hot Jupiter Atmosphere Michael Line ( [email protected] ) Arizona State University https://orcid.org/0000-0001-6247-8323 Matteo Brogi Warwick University Jacob Bean University of Chicago Siddharth Gandhi University of Warwick Joseph Zalesky Arizona State University Viven Parmentier University of Oxford Peter Smith Arizona State University Gregory Mace University of Texas-Austin Megan Manseld University of Chicago Eliza Kempton University of Maryland https://orcid.org/0000-0002-1337-9051 Jonathan Fortney University of California, Santa Cruz Evgenya Shkolnik Arizona State University Jennifer Patience Arizona State University Emily Rauscher University of Michigan https://orcid.org/0000-0003-3963-9672 Jean-Michel Desert University of Amsterdam Jooost Wardenier University of Oxford Physical Sciences - Article Keywords: Gas Giant Planets, Atmospheric Compositions, Giant Planet Formation, Ground-based Spectroscopy, Metallicity Posted Date: June 9th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-593104/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License 1 A Precise Carbon and Oxygen Abundance Determination 2 in a Hot Jupiter Atmosphere 1,2 3,4,5 6 3,4 1 3 Michael R Line , Matteo Brogi , Jacob L. Bean , Siddharth Gandhi , Joseph Zalesky , 7 1 8 9 10 4 Vivien Parmentier , Peter Smith , Gregory N. Mace , Megan Mansfield , Eliza M.-R. Kempton , 11 1,2 1 12 5 Jonathan J. Fortney , Evgenya Shkolnik , Jennifer Patience , Emily Rauscher -

K2-114B and K2-115B: Two Transiting Warm Jupiters
The Astronomical Journal, 154:188 (11pp), 2017 November https://doi.org/10.3847/1538-3881/aa8bb9 © 2017. The American Astronomical Society. All rights reserved. K2-114b and K2-115b: Two Transiting Warm Jupiters Avi Shporer1 , George Zhou2 , Benjamin J. Fulton3,4,24 , Andrew Vanderburg2,24 , Nestor Espinoza5,6 , Karen Collins7 , David Ciardi8, Daniel Bayliss9 , James D. Armstrong10, Joao Bento11, Francois Bouchy9, William D. Cochran12 , Andrew Collier Cameron13, Knicole Colón14 , Ian Crossfield15, Diana Dragomir16,25 , Andrew W. Howard4 , Steve B. Howell17 , Howard Isaacson18 , John F. Kielkopf19, Felipe Murgas20,21, Ramotholo Sefako22, Evan Sinukoff3,4 , Robert Siverd23 , and Stephane Udry9 1 Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125, USA 2 Harvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge, MA 02138, USA 3 Institute for Astronomy, University of Hawaii, Honolulu, HI 96822, USA 4 California Institute of Technology, Pasadena, CA, USA 5 Instituto de Astrofísica, Facultad de Física, Pontificia Universidad Católica de Chile, Av.Vicuña Mackenna 4860, 782-0436 Macul, Santiago, Chile 6 Millennium Institute of Astrophysics, Av.Vicuña Mackenna 4860, 782-0436 Macul, Santiago, Chile 7 Department of Physics & Astronomy, Vanderbilt University, Nashville, TN 37235, USA 8 NASA Exoplanet Science Institute/Caltech, Pasadena, CA, USA 9 Observatoire Astronomique de l’Universit’e de Geneve, 51 ch.des Maillettes, 1290 Versoix, Switzerland 10 University of Hawaii Institute for Astronomy