T-2854 by ARTHUR LARES LIBRARY COLORADO SCHOOL Ot MINES
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Generally View
ISSN 2070-5379 Neftegasovaâ geologiâ. Teoriâ i practika (RUS) URL: http://www.ngtp.ru 1 Morariu D. Petroleum Geologist, Geneva, Switzerland, [email protected] Averyanova O.Yu. All-Russia Petroleum Research Exploration Institute (VNIGRI), Saint Petersburg, Russia, [email protected] SOME ASPECTS OF OIL SHALE - FINDING KEROGEN TO GENERATE OIL Oil demand is predicted to continue to increase despite the high price of oil. The lagging supply increased the prices for oil and gas and a definitive oil replacement has still not been found. Huge oil shale resources discovered in the world, if developed, may increase petroleum supplies. Developing of oil shale needs the availability of low cost production; the greatest risks facing oil shale developing are higher production expenses and lower oil prices. There are several technologies for producing oil from kerogen bearing oil shale, by pyrolysis (heating, retorting). Oil shale is still technologically difficult and expensive to produce and the major impediment is cost. Developing oil shale accumulations means to face huge challenges, but an efficient oil shale development can be accomplished and an acceptable oil shale industry based on new technologies can be built nowadays. Key words: shale, continuous accumulation, oil shale, shale oil, total organic carbon, Rock- Eval, reserves estimation, Fischer assay, mining and retorting, in situ retorting and extraction, in capsule extraction. Introduction Continuous accumulations are extensive petroleum reservoirs not necessarily related to conventional structural or stratigraphic traps [Klett and Charpentier, 2006]. These accumulations lack well defined down-dip petroleum water contacts and these are not localised by the buoyancy of oil or natural gas in water [Schmoker, 1996]. -

Pace Syntfletic Fne Its Report
pace syntfletic fne its report OIL SHALE 0 COAL 0 OIL SANDS VOLUME 25 - NUMBER 4 - DECEMBER 1988 QUARTERLY Tsll Ertl Repository s Library ; Jhoi of M:cs ®THE PACE CONSULTANTS INC. Reg. U.S. P.I. OFF. Pace Synthetic Fuels Report Is published by The Pace Consultants Inc., as a multi-client service and Is intended for the sole use of the clients or organizations affiliated with clients by virtue of a relationship equivalent to 51 percent or greater ownership. Pace Synthetic Fuels Report is protected by the copyright laws of the United States; reproduction of any part of the publication requires the express permission of The Pace Con- sultants Inc. The Pace Consultants Inc., has provided energy consulting and engineering services since 1955. The company's experience includes resource evalua- tion, process development and design, systems planning, marketing studies, licensor comparisons, environmental planning, and economic analysis. The Synthetic Fuels Analysis group prepares a variety of periodic and other reports analyzing developments in the energy field. THE PACE CONSULTANTS INC. SYNTHETIC FUELS ANALYSIS F V.1 I V1t'J 1 1 [tU :4i] j Jerry E. Sinor Pt Office Box 649 Niwot, Colorado 80544 (303) 652-2632 BUSINESS MANAGER Horace 0. Hobbs Jr. Post Office Box 53473 Houston, Texas 77052 (713) 669-7816 Telex: 77-4350 CONTENTS HIGHLIGIrFS A-i I. GENERAL CORPORATIONS Davy Buys Dravo Engineering 1-1 New Zealand Synfuels Plant May Sell Methanol i_I Tenneco Dismembers Its Oil and Gas Operations 1-1 GOVERNMENT Alternative Motor Fuels Act of 1988 Becomes Law 1-2 DOT Develops Alternative Fuels Initiative 1-2 U.S. -

DOE/ER/30013 Prepared for Office of Energy Research, U.S. Department of Energy Agreement No
DOE/ER/30013 prepared for Office of Energy Research, U.S. Department of Energy Agreement No. DE-AC02-81ER30013 SHALE OIL RECOVERY SYSTEMS INCORPORATING ORE BENEFICIATION Final Report, October 1982 by M.A. Weiss, I.V. Klumpar, C.R. Peterson & T.A. Ring Report No. MIT-EL 82-041 Synthetic Fuels Center, Energy Laboratory Massachusetts Institute of Technology Cambridge, MA 02139 NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed or represents that its use would not infringe privately-owned rights. Abstract This study analyzed the recovery of oil from oil shale by use of proposed systems which incorporate beneficiation of the shale ore (that is, concentration of the kerogen) before the oil-recovery step. The objective was to identify systems which could be more attractive than conventional surface retorting of ore. No experimental work was carried out. The systems analyzed consisted of beneficiation methods which could increase kerogen concentrations by at least four-fold. Potentially attractive low-enrichment methods such as density separation were not examined. The technical alternatives considered were bounded by the secondary crusher as input and raw shale oil as output. A sequence of ball milling, froth flotation, and retorting concentrate is not attractive for Western shales compared to conventional ore retorting; transporting the concentrate to another location for retorting reduces air emissions in the ore region but cost reduction is questionable. -

Evaluation of the Potential for Groundwater Transport of Mutagenic Compounds Released by Spent Oil Shale
Utah State University DigitalCommons@USU Reports Utah Water Research Laboratory January 1983 Evaluation of the Potential for Groundwater Transport of Mutagenic Compounds Released by Spent Oil Shale Robert E. Hinchee V. Dean Adams Jeffrey G. Curtis Alberta J. Seierstad Follow this and additional works at: https://digitalcommons.usu.edu/water_rep Part of the Civil and Environmental Engineering Commons, and the Water Resource Management Commons Recommended Citation Hinchee, Robert E.; Adams, V. Dean; Curtis, Jeffrey G.; and Seierstad, Alberta J., "Evaluation of the Potential for Groundwater Transport of Mutagenic Compounds Released by Spent Oil Shale" (1983). Reports. Paper 414. https://digitalcommons.usu.edu/water_rep/414 This Report is brought to you for free and open access by the Utah Water Research Laboratory at DigitalCommons@USU. It has been accepted for inclusion in Reports by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Evaluation Of The Potential For Groundwater Transport Of Mutagenic Compounds Released By Spent Oil Shale Robert E. Hinchee V. Dean Adams Jeffery G. Curtis Alberta J. Seierstad Utah Water Research Laboratory WATER QUALITY SERIES Utah State University UWRL/Q-83/06 Logan, Utah 84322 July 1983 EVALUATION OF THE POTENTIAL FOR GROUNDWATER TRANSPORT OF MUTAGENIC COMPOUNDS RELEASED BY SPENT OIL SHALE by Robert E. Hinchee V. Dean Adams Jeffery G. Curtis Alberta J. Seierstad The research on which this report is based was financed in part by the U. S. Department of the Interior, as authorized by the Water Research and Development Act of 1978 (P.L. 95-467). Project No. B-210-UTAH, Contract No. -

An Assessment of Oil Shale Technologies
An Assessment of Oil Shale Technologies June 1980 NTIS order #PB80-210115 Library of Congress Catalog Card Number 80-600101 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock No. 052-003 -00759-2 Foreword For many decades, the oil shale resources of the Western United States have been considered possible contributors to the Nation’s liquid fuel supply. This vol- ume reviews several paths to development of these resources and the likely conse- quences of following these paths. A chapter providing background information about the nature of oil shale is followed by an evaluation of technologies for recov- ery of shale oil. The economics and finances of establishing an industry of various sizes are analyzed. The fact that much of the best shale is located on Federal land is examined in light of the desire to increase use of the resources. The conse- quences of shale development in terms of impact on the physical and social envi- ronments, and a discussion of the availability of water complete the report. Policy options addressing barriers that could hinder the establishment of the industry are presented. These options, designed primarily for Congressional con- sideration, are limited to the obstacles OTA identified as currently existing. Other issues, of equal importance for the protection of the environment and the commu- nities, but not constraints to development, are discussed in the body of the report. The assessment deals only with oil shale; no systematic attempt was made in this study to compare this energy source with liquid fuel sources other than conven- tional petroleum or with alternative energy strategies. -
? ,<, II I IIIII F
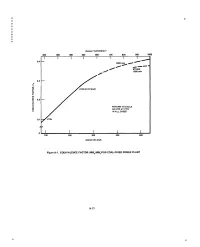
P P P P P P P P P P degrees FAHRENHEIT 200 300 400 500 600 700 800 900 1000 I I i I I I I I 0.4 m__ 3500 psia A f STEAM ~ 1800 psia f 0.3 i .? ,<, 0,2 > RETURN TO CYCLE AS HTW AT 77oc uJ IN ALL CASES 0.1 ~ " HTW 100 200 300 400 500 degrees C E LSI US Figure A-1. EQUIVALENCE FACTOR (MWe/MWt)FOR COAL-FIRED POWER PLANT A-15 P p la la Again, using data from the previous studies, updated to 1/795 and a 1995 date of initial operation for a plant, $51-53/MWh~ is the busbar cost of base load electricity (CF = 0.80) with LWR and coal plants respectively. About 25 percent is added for transmission capital and operating (losses) costs to be more comparable to dispersed generation, giving $65/MWh~. The allocation of costs to electricity and to heat is somewhat arbitrary. Recovery of all costs by the revenues received from sale of both products is the important criterion. Within reason the amount of ther- mal energy extracted can be increased, with a drop in electric output proportional to the equivalence factor. At 0.80 capacity factor the 512 MW~ plant, producing only electricity must recover revenues of $298 M. At base load rates it will receive only $233 M. The shortfall is $65 M. If 300 MW t are extracted, the electric output is reduced to 454 MW~. Selling the heat at $30/MWht and electricity at $65/MWh~ gives an annual revenue of $270 M. -

Assessment of Possible Carcinogenic Hazards Created in Surrounding Ecosystems by Oil Shale Developments
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1976 Assessment of Possible Carcinogenic Hazards Created in Surrounding Ecosystems by Oil Shale Developments Gerald L. Dassler Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Civil and Environmental Engineering Commons Recommended Citation Dassler, Gerald L., "Assessment of Possible Carcinogenic Hazards Created in Surrounding Ecosystems by Oil Shale Developments" (1976). All Graduate Theses and Dissertations. 1559. https://digitalcommons.usu.edu/etd/1559 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. UTAH STATE UNIVERSITY IIII~IIII~I~IIIII~II39060 012144134 ASSESSMENT OF POSSIBLE CARCINOGENIC HAZARDS CREATED IN SURROUNDING ECOSYSTEMS BY OIL SHALE DEVELOPMENTS by Gerald L. Dassler A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Civil and Environmental Engineering Major Professor Committee Member . "" Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 1976 ii ACKNOWLEDGMENTS I wish to express my gratitude to Dr. Don Porcella for the time he spent lending direction and guidance to this report. I also want to thank Dr. V. Dean Adams and Dr. A. Bruce Bishop for their support of my effort. The cooperation and contributions of people within The Oil Shale Corporation (TOSCO), especially Dr. R. Merril Coomes, deserve special recognition and a note of appreciation. -

Water Resources and Potential Hydrologic Effects of Oil-Shale Development in the Southeastern Uinta Basin, Utah and Colorado
f*** k JL-i*-< ;^~<r» \ PUB JU9LUdO|9A9Q pue S90jnos0y Water Resources and Potential Hydrologic Effects of Oil-Shale Development in the Southeastern Uinta Basin, Utah and Colorado By K. L. LINDSKOV and B. A. KIMBALL U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1307 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1984 UNITED STATES DEPARTMENT OF THE INTERIOR WILLIAM P. CLARK, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director Library of Congress Cataloging in Publication Data Lindskov, K. L. Water resources and potential hydrologic effects of oil-shale development in the southeastern Uinta Basin, Utah and Colorado. (Geological Survey professional paper; 1307) Bibliography: 32 p. 1. Oil-shale industry Environmental aspects Uinta Basin (Utah and Colo.) 2. Water- supply Uinta Basin (Utah and Colo.) 3. Hydrology Uinta Basin (Utah and Colo.) I. Kimball, Briant A. II. Title. III. Series. TD195.04L56 1984 338.4*76654 83-600313 For sale by the Branch of Distribution, U.S. Geological Survey, 604 South Picket! Street, Alexandria, VA 22304 CONTENTS Page Page Abstract 1 Selected sources of water supply for an oil-shale industry Continued Introduction - 1 White River Continued Purpose and scope ------------------------------ 1 Regulated flow - 22 Geographic, geologic, and hydrologic setting- 4 White River Dam - Previous investigations - 22 Off-stream storage in Hells Hole Canyon---------- 23 The hydrologic system 9 Green River---------------------------- --------------------- 23 Major rivers --- 9 Ground water - 23 Intra area 9 Potential impacts -

Structural Characterization of Organic Matter in Oil Shales Using Multiple Nuclear Magnetic Resonance Spectroscopic Techniques
Old Dominion University ODU Digital Commons Chemistry & Biochemistry Theses & Dissertations Chemistry & Biochemistry Summer 8-2020 Structural Characterization of Organic Matter in Oil Shales Using Multiple Nuclear Magnetic Resonance Spectroscopic Techniques Wenying Chu Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/chemistry_etds Part of the Analytical Chemistry Commons, and the Geochemistry Commons Recommended Citation Chu, Wenying. "Structural Characterization of Organic Matter in Oil Shales Using Multiple Nuclear Magnetic Resonance Spectroscopic Techniques" (2020). Doctor of Philosophy (PhD), Dissertation, Chemistry & Biochemistry, Old Dominion University, DOI: 10.25777/1bq4-6j64 https://digitalcommons.odu.edu/chemistry_etds/51 This Dissertation is brought to you for free and open access by the Chemistry & Biochemistry at ODU Digital Commons. It has been accepted for inclusion in Chemistry & Biochemistry Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. STRUCTURAL CHARACTERIZATION OF ORGANIC MATTER IN OIL SHALES USING MULTIPLE NUCLEAR MAGNETIC RESONANCE SPECTROSCOPIC TECHNIQUES by Wenying Chu B.S. June 2008, Huazhong Agricultural University, China A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY CHEMISTRY OLD DOMINION UNIVERSITY August 2020 Approved by: Jingdong Mao (Director) Guijun Wang (Member) John R. Donat (Member) Lesley H. Greene (Member) Shizhi Qian (Member) ABSTRACT STRUCTURAL CHARACTERIZATION OF ORGANIC MATTER IN OIL SHALES USING MULTIPLE NUCLEAR MAGNETIC RESONANCE SPECTROSCOPIC TECHNIQUES Wenying Chu Old Dominion University, 2020 Director: Dr. Jingdong Mao Oil shale is a promising source of hydrocarbon fuel that is distributed throughout the world. -

Coo-5 107-1 Predicted Costs Of
COO-5 107-1 PREDICTED COSTS OF ENVIRONMENTAL CONTROLS FOR A COMMERCIAL OIL SHALE INDUSTRY Volume 1 - An Engineering Analysis BY Thomas D. Nevens Charles H. iPrien William J. Culbertson, J r. R. Edwin Hicks , John R. Wallace Ronald F. l’robstein Graham C. Taylor George Dotnahidy Andrew P. J ovanovich July 1979 E Work Performed Under Contract No. EP-78-S-02-5107 Charles H. Prien Center for Oil Shale Research Denver Research Institute Denver, Colorado and Water Purification Associates Cambridge, Massac h uset ts and Stone and Webster Engineering Corporation Denver, Colorado PA DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency Thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. COO-5 107 -1 Distribution Category UC-91 PREDICTED COSTS OF ENVIRONMENTAL CONTROLS FOR A COMMERCIAL OIL SHALE INDUSTRY Volume I An Engineering Analysis Thomas D. -

An Assessment of Oil Shale Technologies
CHAPTER 8 Environmental Considerations Page Page Introduction . ......255 Policy Options for Water Quality Management 314 Summary of Findings . ...................256 Safety and Health. ................315 Air Quality . 256 Introduction . ......315 Water Quality. ........................256 Safety and Health Hazards . ..............316 Occupational Health and Safety . ..........257 Summary of Hazards andTheir Severity. ....322 Land Reclamation. ..258 Federal Laws, Standards, and Regulations. ..324 Permitting. ........................259 Control and Mitigation Methods . ..........325 Summary of Issues and R&D Needs . 326 Air Quality . .. ... ... .+ ...259 Current R&D Programs. .................326 Introduction . ......259 Policy Considerations. ..................327 Pollutant Generation . ..................259 Air Quality Laws, Standards, and Regulations 263 Land Reclamation. .....................328 Air Pollution Control Technologies . ........269 Introduction . ......328 Pollutant Emissions. .278 Reasons for Reclamation . ................329 Dispersion Modeling. ...................279 Regulations Governing Land Reclamation. ...329 A Summary of Issues and Policy Options. ....288 Reclamation Approaches. ...............330 The Physical and Chemical Characteristics Water Quality. ........................291 of Processed Shale . .. ....332 Introduction . 29l Use of Topsoil as a Spent Shale Cover . ......335 Pollution Generation. 292 Species Selection and Plant Materials . 336 Summary of Pollutants Produced by Major Review of Selected Research -

California State University, Northridge Oil Shale: An
CALIFORNIA STATE UNIVERSITY, NORTHRIDGE OIL SHALE: AN OVERVIEW ) ( A graduate project submitted in partial satisfaction of the requirements for the degree of Master of Science 1n Engineering by John Page ------- June, 1979 The Graduate Project of John Page is approved: Jon H. California State University, Northridge ii PREFACE This report deals with various aspects of the oil shale industry in the United States today. Only that part of the industry which relates to above ground retorting is focused on. In situ technology will not be discussed as such, but will be included only insofar as it adds con- tinuity to this report. The in situ process is not focused on because it is still largely in the study phase, is not yet technically ready for commercialization, and forms a small part of the overall oil shale industry. There is much published literature which focuses on particular facets of oil shale. One can find information on retorting, costs, environmental aspects, and a host of other specialized topics. However, works which treat oil shale on the whole are few. It is the purpose of this report to fill such a gap; to give the reader an up-to-date overview of the salient aspects of the technology, problems and prospects for the industry. ].ii TABLE OF CONTENTS _DPaae__ PREFACE iii LIST OF TABLES vi LIST OF FIGURES viii ABSTRACT ix Chapter I RESERVES 1 II RETORTING 6 Bureau of Mines Gas-Combustion Retort 7 Union Oil Company Process 17 The TOSCO-II Process 21 The Paraho Process 24 III REFINING 30 Union Oil Company 30 Applied Systems Corporation