Identification of Biomolecules Involved in the Adaptation to The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metaproteomics As a Tool for Studying the Protein Landscape of Human-Gut Bacterial Species
bioRxiv preprint doi: https://doi.org/10.1101/2021.09.02.458484; this version posted September 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Metaproteomics as a tool for studying the protein landscape of human-gut bacterial species Moses Stamboulian1, Jamie Canderan1, and Yuzhen Ye1 1Luddy School of Informatics, Computing and Engineering, Indiana University, 700 N. Woodlawn Avenue, Bloomington, IN 47408. Email address: Moses Stamboulian, [email protected] Jamie Canderan, [email protected] Yuzhen Ye, [email protected] Corresponding author: Yuzhen Ye, [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.09.02.458484; this version posted September 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Host-microbiome interactions and the microbial community have broad impact in human health and diseases. Most microbiome based studies are performed at the genome level based on next-generation sequencing techniques, but metaproteomics is emerging as a pow- erful technique to study microbiome functional activity by characterizing the complex and dynamic composition of microbial proteins. We conducted a large-scale survey of human gut microbiome metaproteomic data to identify generalist species that are ubiquitously expressed across all samples and specialists that are highly expressed in a small subset of samples associ- ated with a certain phenotype. -
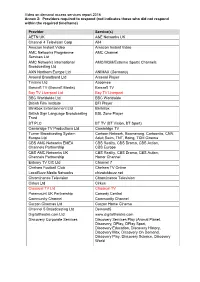
Annex 2: Providers Required to Respond (Red Indicates Those Who Did Not Respond Within the Required Timeframe)
Video on demand access services report 2016 Annex 2: Providers required to respond (red indicates those who did not respond within the required timeframe) Provider Service(s) AETN UK A&E Networks UK Channel 4 Television Corp All4 Amazon Instant Video Amazon Instant Video AMC Networks Programme AMC Channel Services Ltd AMC Networks International AMC/MGM/Extreme Sports Channels Broadcasting Ltd AXN Northern Europe Ltd ANIMAX (Germany) Arsenal Broadband Ltd Arsenal Player Tinizine Ltd Azoomee Barcroft TV (Barcroft Media) Barcroft TV Bay TV Liverpool Ltd Bay TV Liverpool BBC Worldwide Ltd BBC Worldwide British Film Institute BFI Player Blinkbox Entertainment Ltd BlinkBox British Sign Language Broadcasting BSL Zone Player Trust BT PLC BT TV (BT Vision, BT Sport) Cambridge TV Productions Ltd Cambridge TV Turner Broadcasting System Cartoon Network, Boomerang, Cartoonito, CNN, Europe Ltd Adult Swim, TNT, Boing, TCM Cinema CBS AMC Networks EMEA CBS Reality, CBS Drama, CBS Action, Channels Partnership CBS Europe CBS AMC Networks UK CBS Reality, CBS Drama, CBS Action, Channels Partnership Horror Channel Estuary TV CIC Ltd Channel 7 Chelsea Football Club Chelsea TV Online LocalBuzz Media Networks chizwickbuzz.net Chrominance Television Chrominance Television Cirkus Ltd Cirkus Classical TV Ltd Classical TV Paramount UK Partnership Comedy Central Community Channel Community Channel Curzon Cinemas Ltd Curzon Home Cinema Channel 5 Broadcasting Ltd Demand5 Digitaltheatre.com Ltd www.digitaltheatre.com Discovery Corporate Services Discovery Services Play -

Configuration Manager User Guide © 2019 Quest Software Inc
Quest® Enterprise Reporter 3.2.1 Configuration Manager User Guide © 2019 Quest Software Inc. ALL RIGHTS RESERVED. This guide contains proprietary information protected by copyright. The software described in this guide is furnished under a software license or nondisclosure agreement. This software may be used or copied only in accordance with the terms of the applicable agreement. No part of this guide may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying and recording for any purpose other than the purchaser’s personal use without the written permission of Quest Software Inc. The information in this document is provided in connection with Quest Software products. No license, express or implied, by estoppel or otherwise, to any intellectual property right is granted by this document or in connection with the sale of Quest Software products. EXCEPT AS SET FORTH IN THE TERMS AND CONDITIONS AS SPECIFIED IN THE LICENSE AGREEMENT FOR THIS PRODUCT, QUEST SOFTWARE ASSUMES NO LIABILITY WHATSOEVER AND DISCLAIMS ANY EXPRESS, IMPLIED OR STATUTORY WARRANTY RELATING TO ITS PRODUCTS INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. IN NO EVENT SHALL QUEST SOFTWARE BE LIABLE FOR ANY DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE, SPECIAL OR INCIDENTAL DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS, BUSINESS INTERRUPTION OR LOSS OF INFORMATION) ARISING OUT OF THE USE OR INABILITY TO USE THIS DOCUMENT, EVEN IF QUEST SOFTWARE HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. Quest Software makes no representations or warranties with respect to the accuracy or completeness of the contents of this document and reserves the right to make changes to specifications and product descriptions at any time without notice. -

A Decade of Metaproteomics: Where We Stand and What the Future Holds
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Repository and Bibliography - Luxembourg www.proteomics-journal.com Page 1 Proteomics Viewpoint A decade of metaproteomics: where we stand and what the future holds Paul Wilmes1, Anna Heintz-Buschart1 and Philip L. Bond2 1Luxembourg Centre for Systems Biomedicine, University of Luxembourg, Esch-sur-Alzette, Luxembourg 2Advanced Water Management Centre, University of Queensland, Brisbane, Australia Correspondence: Paul Wilmes, Luxembourg Centre for Systems Biomedicine, University of Luxembourg, 7 avenue des Hauts-Fourneaux, L-4362 Esch-sur-Alzette, Luxembourg; Philip L. Bond, Advanced Water Management Centre, University of Queensland, Queensland 4072, Australia E-mail: [email protected]; [email protected] Fax: (352) 466644 6188; (61) 07 33654726 Received: 15-May-2015; Revised: 06-Jul-2015; Accepted: 05-Aug-2015. This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/pmic.201500183. This article is protected by copyright. All rights reserved. www.proteomics-journal.com Page 2 Proteomics Abstract We are living through exciting times during which we are able to unravel the “microbial dark matter” in and around us through the application of high-resolution “meta-omics”. Metaproteomics offers the ability to resolve the major catalytic units of microbial populations and thereby allows the establishment of genotype-phenotype linkages from in situ samples. A decade has passed since the term “metaproteomics” was first coined and corresponding analyses were carried out on mixed microbial communities. -

And Metaproteomics-Based Rapid Assay of Individual 2 Microbiome Responses to Drugs
bioRxiv preprint doi: https://doi.org/10.1101/543256; this version posted June 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 RapidAIM: A culture- and metaproteomics-based Rapid Assay of Individual 2 Microbiome responses to drugs 3 4 Authors: Leyuan Li1, Zhibin Ning1, Xu Zhang1, Janice Mayne1, Kai Cheng1, Alain Stintzi*1, 5 Daniel Figeys*1,2,3 6 Affiliations: 7 1 Department of Biochemistry, Microbiology and Immunology, Ottawa Institute of Systems Biology, Faculty of 8 Medicine, University of Ottawa, Ottawa, Canada. 9 2 Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, Canada. 10 3 Canadian Institute for Advanced Research, Toronto, Canada. 11 *To whom correspondence should be addressed: Emails: [email protected] (DF), and [email protected] (AS). 12 13 Abstract: 14 Background: Human-targeted drugs may exert off-target effects on the gut microbiota. 15 However, our understanding of such effects is limited due to a lack of rapid and scalable assay to 16 comprehensively assess microbiome responses to drugs. Drugs can drastically change the overall 17 microbiome abundance, microbial composition and functions of a gut microbiome. Although we 18 could comprehensively observe these microbiome responses using a series of tests, for the 19 purpose of a drug screening, it is important to decrease the number of analytical tools used. 20 Results: Here, we developed an approach to screen compounds against individual microbiomes 21 in vitro using metaproteomics adapted for both absolute bacterial abundances and functional 22 profiling of the microbiome. -
Channel Guide August 2018
CHANNEL GUIDE AUGUST 2018 KEY HOW TO FIND WHICH CHANNELS YOU HAVE 1 PLAYER PREMIUM CHANNELS 1. Match your ENTERTAINMENT package 1 2 3 4 5 6 2 MORE to the column 100 Virgin Media Previews 3 M+ 101 BBC One If there’s a tick 4 MIX 2. 102 BBC Two in your column, 103 ITV 5 FUN you get that 104 Channel 4 6 FULL HOUSE channel ENTERTAINMENT SPORT 1 2 3 4 5 6 1 2 3 4 5 6 100 Virgin Media Previews 501 Sky Sports Main Event 101 BBC One HD 102 BBC Two 502 Sky Sports Premier 103 ITV League HD 104 Channel 4 503 Sky Sports Football HD 105 Channel 5 504 Sky Sports Cricket HD 106 E4 505 Sky Sports Golf HD 107 BBC Four 506 Sky Sports F1® HD 108 BBC One HD 507 Sky Sports Action HD 109 Sky One HD 508 Sky Sports Arena HD 110 Sky One 509 Sky Sports News HD 111 Sky Living HD 510 Sky Sports Mix HD 112 Sky Living 511 Sky Sports Main Event 113 ITV HD 512 Sky Sports Premier 114 ITV +1 League 115 ITV2 513 Sky Sports Football 116 ITV2 +1 514 Sky Sports Cricket 117 ITV3 515 Sky Sports Golf 118 ITV4 516 Sky Sports F1® 119 ITVBe 517 Sky Sports Action 120 ITVBe +1 518 Sky Sports Arena 121 Sky Two 519 Sky Sports News 122 Sky Arts 520 Sky Sports Mix 123 Pick 521 Eurosport 1 HD 132 Comedy Central 522 Eurosport 2 HD 133 Comedy Central +1 523 Eurosport 1 134 MTV 524 Eurosport 2 135 SYFY 526 MUTV 136 SYFY +1 527 BT Sport 1 HD 137 Universal TV 528 BT Sport 2 HD 138 Universal -

REPORT CARD: MAKING the HDTV PLUNGE by RON HRANAC
Originally appeared in the March 2009 issue of Communications Technology. REPORT CARD: MAKING THE HDTV PLUNGE By RON HRANAC After putting it off for several years, I finally joined the slightly more than one-third of Americans who have already done so, and got a high definition TV (HDTV) set for home use. I had all sorts of excuses for putting this off, among them cost, a 15+ year-old conventional TV set that still works fine, a wife who said the 15+ year-old TV set works just fine (see a trend here?), picture quality with standard definition (SD) sources, rear projection vs. liquid crystal display (LCD) vs. plasma, and even picture quality with HD sources. During the past few years, the cost of HD sets has come down dramatically. Technology has improved to the point where both SD and HD picture quality are quite good on many of the new sets, though there are still some HD sets that do an abysmal job displaying SD material. The latter is important because, quite frankly, a lot of video content is SD and will be for the foreseeable future. Then and now My first experience with HDTV goes back to a 1980s National Association of Broadcasters convention. The concept of high-def had been introduced as a way to provide widescreen TV viewing rivaling projected 35 mm motion pictures, accompanied by CD-quality audio. One of the hopes was that a global HDTV standard could be developed, avoiding the NTSC/PAL/SECAM format battles that have long plagued broadcast TV - OK, maybe that was wishful thinking. -

Globalization Strategies Utilized by Discovery Networks: a Case Study
Globalization Strategies Utilized by Discovery Networks: A Case Study A Thesis Submitted to the Faculty of Drexel University by Jingyi Yang in partial fulfillment of the requirements for the degree of Master of Science February 2014 ii iii © Copyright 2014 Jingyi Yang. All Rights Reserved. iv Table of Contents Table of Contents .................................................................................................. iv List of Tables ......................................................................................................... v CHAPTER 1: INTRODUCTION .......................................................................... 1 Introduction ................................................................................................................... 1 Purpose of the Study ..................................................................................................... 3 Statement of the Problem .............................................................................................. 4 Research Questions ....................................................................................................... 7 Definitions ..................................................................................................................... 8 Limitations .................................................................................................................. 10 CHAPTER 2: LITERATURE REVIEW ............................................................. 11 CHAPTER 3: METHODOLOGY ...................................................................... -

TV & Radio Channels Astra 2 UK Spot Beam
UK SALES Tel: 0345 2600 621 SatFi Email: [email protected] Web: www.satfi.co.uk satellite fidelity Freesat FTA (Free-to-Air) TV & Radio Channels Astra 2 UK Spot Beam 4Music BBC Radio Foyle Film 4 UK +1 ITV Westcountry West 4Seven BBC Radio London Food Network UK ITV Westcountry West +1 5 Star BBC Radio Nan Gàidheal Food Network UK +1 ITV Westcountry West HD 5 Star +1 BBC Radio Scotland France 24 English ITV Yorkshire East 5 USA BBC Radio Ulster FreeSports ITV Yorkshire East +1 5 USA +1 BBC Radio Wales Gems TV ITV Yorkshire West ARY World +1 BBC Red Button 1 High Street TV 2 ITV Yorkshire West HD Babestation BBC Two England Home Kerrang! Babestation Blue BBC Two HD Horror Channel UK Kiss TV (UK) Babestation Daytime Xtra BBC Two Northern Ireland Horror Channel UK +1 Magic TV (UK) BBC 1Xtra BBC Two Scotland ITV 2 More 4 UK BBC 6 Music BBC Two Wales ITV 2 +1 More 4 UK +1 BBC Alba BBC World Service UK ITV 3 My 5 BBC Asian Network Box Hits ITV 3 +1 PBS America BBC Four (19-04) Box Upfront ITV 4 Pop BBC Four (19-04) HD CBBC (07-21) ITV 4 +1 Pop +1 BBC News CBBC (07-21) HD ITV Anglia East Pop Max BBC News HD CBeebies UK (06-19) ITV Anglia East +1 Pop Max +1 BBC One Cambridge CBeebies UK (06-19) HD ITV Anglia East HD Psychic Today BBC One Channel Islands CBS Action UK ITV Anglia West Quest BBC One East East CBS Drama UK ITV Be Quest Red BBC One East Midlands CBS Reality UK ITV Be +1 Really Ireland BBC One East Yorkshire & Lincolnshire CBS Reality UK +1 ITV Border England Really UK BBC One HD Channel 4 London ITV Border England HD S4C BBC One London -

A Community Proposal to Integrate Proteomics Activities In
F1000Research 2017, 6:875 Last updated: 05 OCT 2021 OPINION ARTICLE A community proposal to integrate proteomics activities in ELIXIR [version 1; peer review: 2 approved] Juan Antonio Vizcaíno 1, Mathias Walzer 1, Rafael C. Jiménez 2, Wout Bittremieux3, David Bouyssié4, Christine Carapito4, Fernando Corrales5, Myriam Ferro4, Albert J.R. Heck6,7, Peter Horvatovich 8, Martin Hubalek9, Lydie Lane10,11, Kris Laukens3, Fredrik Levander12, Frederique Lisacek13,14, Petr Novak15, Magnus Palmblad 16, Damiano Piovesan17, Alfred Pühler18, Veit Schwämmle 19, Dirk Valkenborg20-22, Merlijn van Rijswijk 23,24, Jiri Vondrasek9, Martin Eisenacher25, Lennart Martens26,27, Oliver Kohlbacher 28-31 1European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Cambridge, CB10 1SD, UK 2ELIXIR Hub, Cambridge, CB10 1SD, UK 3Department of Mathematics and Computer Science, University of Antwerp, Antwerp, 2020, Belgium 4French Proteomics Infrastructure ProFI, Grenoble, (EDyP U1038, CEA/Inserm/ Grenoble Alpes University) Toulouse (IPBS, Université de Toulouse, CNRS, UPS), Strasbourg (LSMBO, IPHC UMR7178, CNRS-Université de Strasbourg), France 5ProteoRed, Proteomics Unit, Centro Nacional de Biotecnología (CSIC), Madrid, 28049, Spain 6Biomolecular Mass Spectrometry and Proteomics, Bijvoet Centre for Biomolecular Research and Utrecht Institute for Pharmaceutical Sciences, University of Utrecht, Utrecht, 3548 CH, The Netherlands 7Netherlands Proteomics Center, Utretcht, 3584 CH, The Netherlands 8Analytical Biochemistry, Department of Pharmacy, -

Gut Microbiome’ on 7Th & 8Th June 2018 in Brussels
THE ESSENTIAL ROLES OF CARBOHYDRATES IN PROMOTING GUT MICROBIOTA FUNCTION THROUGH ALL STAGES OF LIFE This paper was co-authored by Nathalie Juge†, Sabine Flitsch‡, Rodney Townsend§ and Claire Doherty‡ using input from group discussions that took place at the CarboMet workshop ‘The Role of Carbohydrates in the Gut Microbiome’ on 7th & 8th June 2018 in Brussels. CarboMet (Metrology of Carbohydrates for European Bioindustries) is a four-year Coordination and Support Action (CSA) funded by Horizon 2020 FET-OPEN (Grant agreement no. 737395). https://carbomet.eu/) The primary aim of the CSA is to mobilise the European academic and industrial community to identify generic measurement, data management and metrological challenges that must be met in order to advance and exploit carbohydrate knowledge and applications. The potential for exploitation of carbohydrates lies in their diversity and structural complexity – subtle changes in the three-dimensional structure of a carbohydrate profoundly affects (for example) its ability to protect against or fight infectious disease. However, these subtle structural differences present a challenge for their analysis. Sophisticated measurement and metrological capabilities for analysing carbohydrates are available but are nowhere near as advanced or as routinely used in other areas such as gene sequencing. Therefore as a first stage CarboMet has organised some open, Europe-wide workshops to identify key topics where our understanding needs to be advanced urgently, and where current limitations in our measurement, data management and metrological capabilities are hindering progress. The Workshops were also asked to recommend appropriate Work Programmes that should be supported by Horizon 2020 and its successor, Horizon Europe. -

ANNUAL REPORT Marine Science Institute UC SANTA BARBARA Table of Contents
2018–2019 ANNUAL REPORT Marine Science Institute UC SANTA BARBARA Table of Contents 3 Mission Statement 4 From the Director Overview 5 Executive Summary 7 10 Organizational Charts Administrative Staff 11 Centers and Units 12 13 MSI Advisory Committee, Administrative & Technical Staff 16 Statistical Summary Research Support Summary 17 Statistical Summary 2018–2019 19 Five-Year Research Support 21 Summary Funding Agencies 22 24 Principal Investigators 30 Postdoctoral Researchers, Graduate and Undergraduate Students 33 Space 39 Other Projects & Activities Coastal Research Center 40 Marine Biotechnology Center 42 Ocean & Coastal Policy Center 52 Analytical Laboratory 54 Education and Outreach 55 56 Awards Administered Awards 57 Research Summaries 66 2 Mission Statement The Marine Science Institute at the University of California, Santa Barbara, is committed to fostering innovative and significant research, to promoting effective stewardship, and to sharing exciting discoveries of the world’s oceans. 3 From the Director 4 Overview The Marine Science Institute (MSI) provides an intellectual and physical environment at UCSB that fosters world-renowned marine research. The institute brings together marine researchers from across the UCSB campus and supports multi-investigator collaborative projects and individual research efforts. The scientific membership at MSI consists of both ladder faculty and professional researchers. In 2018-2019 MSI membership included 25 ladder faculty and 32 professional researchers with 228 additional participants distributed across postdoctoral scholars, graduate students and undergraduates. Beyond research, MSI’s Research Experience and Education Facility (REEF) educates UCSB students and the general public about MSI science. MSI is housed in the marine science research building (MSRB) on the UCSB campus.