Travel Clinic Operations Guide Edition 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lshtm-Prospectus.Pdf
Welcome The London School of Hygiene & Tropical Medicine (LSHTM) is widely recognised as a world-leading school of public and global health, working closely with partners in the UK and worldwide to address contemporary and future critical health challenges. Our commitment to improving Professor Peter Piot KCMG FMedSci health in the UK and worldwide is the lifeblood of LSHTM, and I am proud Peter Piot is the Director and Handa Contents of the work our staff, students and Professor of Global Health at the alumni do to address major health London School of Hygiene & Tropical inequalities and challenges. Medicine. Professor Piot was part of a team that co-discovered the Ebola INTRODUCTION 03 COURSES 16 We have a diverse and truly global community dedicated virus in 1976, and was a leading voice to quality cross-disciplinary research. LSHTM is involved during the recent Ebola outbreak in at every stage of the research pipeline, from basic science West Africa. He also instigated some Welcome 03 Clinical Trials 16 all the way through to evaluation of health interventions, of the first research programmes Why Choose LSHTM? 04 Epidemiology 18 providing a firm foundation of evidence for improving health. into HIV/AIDS, and was the founding Crucially, we take research out into the real world to make Executive Director of UNAIDS and Why Distance Learning? 05 Global Health Policy 20 a tangible difference in people’s lives. Under-Secretary-General of the LSHTM and University of London 06 Infectious Diseases 22 United Nations from 1995 until 2008. This year marks our School’s 120th anniversary and we Our Global Student Community 08 Public Health 24 are celebrating 120 years of health innovation at LSHTM. -

Introduction to Travel Medicine
1 Introduction to Travel Medicine Phyllis E. Kozarsky and Jay S. Keystone Each year the World Tourism Organization (WTO) publishes its statistics With the fourth edition of the textbook Travel Medicine, the editors revealing staggering numbers of people crisscrossing the globe; indeed, needed to be cognizant of the growth of the body of knowledge over the last decade there have been double-digit increases in travel. (www.istm.org) in the field, while respecting the need to focus content International tourist arrivals reported by the WTO in 2016 grew to on what is most important for the provider to understand practicing 1235 million, 46 million greater than in 2015. Preliminary data show pretravel health. In addition, we have tried to include information the Asia-Pacific region leading the way with 8% growth, the Americas concerning the more common issues facing travelers at their desti- (primarily South and Central America) with 4% growth, and Europe nations as well as on return, being sure to capture the most recent with 2% growth, primarily in the north. Existing data from Africa show developments. a healthy increase in travel to the sub-Saharan region—8% as well. The Because travel is no longer just associated with tourism, but often Middle East has seen a decrease in about 4%. Despite this continued incorporates work, volunteerism, medical care, migration, etc., new growth and despite 2017 having been designated by the United Nations content has also been added to assist the provider in caring for specific as the “International Year of Sustainable Tourism for Development,” populations engaging in different types of travel. -

Ctropmed® Examination)
Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Health (CTropMed® Examination) Striving for Professional Excellence in Clinical Tropical Medicine and Travelers’ Health October 11–24, 2021 NEW IN 2021! The CTropMed® Examination is being offered globally through secure internet-based testing sites. Visit the list of testing centers here. Download Sample Exam Questions #TropMed21 Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Health (CTropMed® Examination) New in 2021! The CTropMed® Examination is being offered globally through secure internet-based testing sites. Visit the list of testing centers here. Table of Contents Fostering professional development in the fields of clinical tropical medicine and Requirements................. 3 travelers’ health is one of the Society’s highest priorities. To that end, ASTMH About the Examination .......... 5 developed the Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Applying for the Examination ..... 5 Health (CTropMed® Examination) as a means to distinguish individuals who have Testing Centers ............... 6 demonstrated advanced knowledge and experience in these fields. The Certificate Test Center Admission of Knowledge in Clinical Tropical Medicine and Travelers’ Health is conferred on Requirements................. 7 Prohibited Items............... 8 1) licensed healthcare professionals applying via Practice Pathway or those Cancellation and healthcare professionals (regardless of licensure) who have passed an ASTMH- Rescheduling Policy............ 9 accredited Diploma Course, 2) have direct experience in the fields of tropical Scoring ..................... 10 medicine and/or travelers’ health, and 3) have passed the ASTMH Examination in Examination Results............ 10 Clinical Tropical Medicine and Travelers’ Health. Special Circumstances.......... 10 ASTMH’s Committment to Inclusion and Respect .......... 11 To support this process, ASTMH accredits specific diploma courses that meet Examination Checklist ......... -
Programme Specification
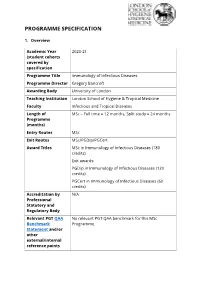
PROGRAMME SPECIFICATION 1. Overview Academic Year 2020-21 (student cohorts covered by specification Programme Title Immunology of Infectious Diseases Programme Director Gregory Bancroft Awarding Body University of London Teaching Institution London School of Hygiene & Tropical Medicine Faculty Infectious and Tropical Diseases Length of MSc – Full time = 12 months, Split study = 24 months Programme (months) Entry Routes MSc Exit Routes MSc/PGDip/PGCert Award Titles MSc in Immunology of Infectious Diseases (180 credits) Exit awards: PGDip in Immunology of Infectious Diseases (120 credits) PGCert in Immunology of Infectious Diseases (60 credits) Accreditation by N/A Professional Statutory and Regulatory Body Relevant PGT QAA No relevant PGT QAA benchmark for this MSc Benchmark Programme. Statement and/or other external/internal reference points Level of programme Masters (MSc) Level 7 within the Framework for Higher Education Qualifications (FHEQ) Total Credits CATS: 180 ECTS: 90 HECoS Code(s) 100345:100265:100948 (1:1:1) Mode of Delivery For 2020-21, the delivery of LSHTM teaching has been adjusted in response to the COVID-19 pandemic. For MSc Immunology of Infectious Diseases: Term 1 core module teaching will be delivered online only, with a combination of synchronous (live and interactive) and asynchronous (recordings, independent study, individual exercises, etc) activities. Terms 2 modules will be offered both online and also on-campus where possible. Some on-campus modules will use a blended approach of both face to face and online delivery if permitted under UK social distancing rules at that time. Term 3 will include on-campus pre- project lab training and access for students undertaking lab-based projects. -

Tourism and the Health Effects of Infectious Diseases: Are There Potential Risks for Tourists?
International Journal of Safety and Security in Tourism/Hospitality Tourism and the Health Effects of Infectious Diseases: Are There Potential Risks for Tourists? David Mc.A Baker Facultad de Ciencias Económicas 1 Tourism and the Health Effects of Infectious Diseases: Are There Potential Risks for Tourists? David Mc.A Baker1 Abstract According to statistics of the WorldTourism Organization (WTO), international touristarrivals for 2014 exceeded 1138 million. In the same year the majority ofinternational tourist arrivals were for thepurposes of leisure, recreation and holiday, about 55%. However travel is a potent force in the emergenceof disease since the migration of humans has beenthe pathway for disseminating infectious diseases throughout recorded history andwill continue to shape the emergence, frequency, and spread of infections in geographicareas and populations. The current volume, speed, and reach of travel are unprecedented.The consequences of travel extend beyond the traveler to the population visitedand the ecosystem.International travel and tourism can pose various risks tohealth, depending on the characteristics ofboth the traveler and the travel. Travelersmay encounter serious health risks that may arise inareas where accommodation is of poorquality, hygiene and sanitation areinadequate.The purpose of this paper is to highlightthe details and impact of significant infectious diseases that can pose a risk to tourists and threaten public health.The paper also seeks to raiseawareness of the issues outlined and thereby increase efforts to enhance travel safety. Keywords: tourism, risks, infectious diseases, health 1. Introduction Recovery from the global financial crisis and an emergence of new source markets has led to considerable growth in the global tourism industry from 2009 to 2014. -

Traveling Outside the United States? Get Vaccinated. Vaccines Protect You from Diseases Not Often Found in the United States
Traveling outside the United States? Get vaccinated. Vaccines protect you from diseases not often found in the United States. Plan ahead for safe, healthy travel. Get vaccinated 2 months before your trip. • Contact your healthcare provider or a travel clinic or pharmacy below. • Vaccines take time to fully protect you. • You may need multiple doses of vaccine, up to 4 weeks apart. Some travelers have special needs to consider. General information may not apply to your health situation or travel plans. You may need to schedule a consultation to determine how you should prepare for safe travel. Especially if you are: • Older than 65. • Traveling with a child younger than 2. • Pregnant. • Living with a medical condition. Online resources • Taking certain medications. Call before you go to Travel Health Online verify services and fees. tripprep.com Travel clinics Centers for Disease Control and Prevention Travelers’ Health Passport Health Virginia Mason Travel Clinic cdc.gov/travel 4301 S. Pine St., Suite 27 33501 First Way S. United States Department of State Tacoma, 98409 Federal Way, 98003 Travel Information (206) 414-2709 (206) 583-6585 travel.state.gov passporthealthusa.com virginiamason.org/travel-health World Health Organization International Travel and Health Pharmacies who.int/health-topics/travel-and-health International Society of Travel Medicine Rite Aid Fred Meyer istm.org Gig Harbor....(253) 851-6939 Bonney Lake ........(253) 891-7333 American Journal of Tropical Medicine Lakewood ....(253) 588-3666 Puyallup................(253) 840-8183 and Hygiene Tacoma .........(253) 474-0115 Sumner .................(253) 826-8433 ajtmh.org Tacoma .................(253) 475-6073 Safeway University Place ...(253) 460-4033 Milton ...........(253) 952-0390 Puyallup........(253) 841-1534 Tacoma .........(253) 566-9217. -

Medical Tourism
Medical Tourism The term medical tourism refers to two distinct, both fairly recent, phenomena: (1) physicians and medical trainees from developed countries who travel to less developed countries to provide medical care, and (2) patients, generally from more developed countries, who travel to less developed countries seeking less expensive medical care or medical procedures (including transplantations) that are unavailable or illegal in their countries of origin. The first type of medical tourism cynically refers to those medical practitioners from Western countries who travel to developing countries for short periods of time to provide medical services, usually for free. While there are no strict criteria that distinguish medical tourists from medical or humanitarian aid workers, medical tourists often have little experience in developing world settings and often combine medical visits with recreational or cultural activities. Although medical tourists are often motivated by genuine altruism, they often overestimate the need for their assistance or the utility of their specific skill set to problems they encounter. Medical tourists may bring much needed medical supplies or expertise, but they may also inadvertently undermine local health-care infrastructure or provide inappropriate, incorrect, Medical Tourism refers to or even harmful medical care. patients who travel abroad for procedures that can cost much more in the United States. Medical trainees, both students and postgraduate residents are increasingly seeking formal or elective experiences internationally. Reasons for this include greater flexibility in medical programs, recognition of the value of international experiences, ease of travel, and résumé building. Many medical trainees are interested in developing their technical or improvisational skills in a more challenging setting, while others are interested in observing other medical systems, alternative and traditional therapies, or learning about cross-cultural issues in medicine. -

MEDICAL GUIDELINES for AIRLINE TRAVEL 2Nd Edition
MEDICAL GUIDELINES FOR AIRLINE TRAVEL 2nd Edition Aerospace Medical Association Medical Guidelines Task Force Alexandria, VA VOLUME 74 NUMBER 5 Section II, Supplement MAY 2003 Medical Guidelines for Airline Travel, 2nd Edition A1 Introduction A1 Stresses of Flight A2 Medical Evaluation and Airline Special Services A2 Medical Evaluation A2 Airline Special Services A3 Inflight Medical Care A4 Reported Inflight Illness and Death A4 Immunization and Malaria Prophylaxis A5 Basic Immunizations A5 Supplemental Immunizations A5 Malaria Prophylaxis A6 Cardiovascular Disease A7 Deep Venous Thrombosis A8 Pulmonary Disease A10 Pregnancy and Air Travel A10 Maternal and Fetal Considerations A11 Travel and Children A11 Ear, Nose, and Throat A11 Ear A11 Nose and sinuses A12 Throat A12 Surgical Conditions A13 Neuropsychiatry A13 Neurological A13 Psychiatric A14 Miscellaneous Conditions B14 Air Sickness B14 Anemia A14 Decompression Illness A15 Diabetes A16 Jet Lag A17 Diarrhea A17 Fractures A18 Ophthalmological Conditions A18 Radiation A18 References Copyright 2003 by the Aerospace Medical Association, 320 S. Henry St., Alexandria, VA 22314-3579 The paper used in this publication meets the minimum requirements of American National Standard for Information Sciences—Permanence of Paper for Printed Library Materials. ANSI Z39.48-1984. Medical Guidelines for Airline Travel, 2nd ed. Aerospace Medical Association, Medical Guidelines Task Force, Alexandria, VA Introduction smoke, uncomfortable temperatures and low humidity, jet lag, and cramped seating (64). Nevertheless, healthy Each year approximately 1 billion people travel by air passengers endure these stresses which, for the most on the many domestic and international airlines. It has part, are quickly forgotten once the destination is been predicted that in the coming two decades, the reached. -

Glossary of Acronyms
Glossary of Acronyms Ab Antibody AFRO World Health Organization Regional Office for Africa ALB, Alb Albendazole AMRO World Health Organization Regional Office for the Americas (see PAHO) APOC World Health Organization African Programme for Onchocerciasis Control BICO Blantyre Institute for Community Opthamology BMGF Bill & Melinda Gates Foundation CAR Central African Republic CCA Circulating Cathodic Antigen CDC U.S. Centers for Disease Control & Prevention CRFilMT Center for Research on Filariasis and Other Tropical Diseases CWW Children Without Worms, The Task Force for Global Health DEC Diethylcarbamazine DOLF Death to Onchocerciasis and Lymphatic Filariasis Project, Washington University in St. Louis DRC Democratic Republic of Congo ELISA Enzyme-Linked Immunosorbent Assay EMRO World Health Organization Regional Office for the Eastern Mediterranean EURO World Health Organization Regional Office for Europe FHI, FHI360 Family Health International FPSU Filarial Programmes Support Unit, Liverpool School of Tropical Medicine (formerly CNTD) FTS Alere Filariasis Test Strip GSK GlaxoSmithKline GTMP Global Trachoma Mapping Project HDI Health and Development International HKI Helen Keller International IBB Institut de Bouisson Bertrand ICT Immunochromatography Test IRD Institut de Recherche pour le Développement ITI International Trachoma Initiative, The Task Force for Global Health IVM, Iver Ivermectin (Generic Name for Mectizan) JCU James Cook University JHU John Hopkins University KEMRI Kenya Medical Research Institute LF Lymphatic Filariasis -

The Educational Background for the Practice of Tropical Medicine
THE EDUCATIONAL BACKGROUND FOR THE PRAC-. TICE OF TROPICAL MEDICINE' FREDERICK F. RUSSELL From the International Health Board, Rockefeller Foundation, New York, N. Y. Received for publication November 14, 1934 My address wifi be brief, as I have really a very simple theme and it will not take much of your time for me to present it. It is unnecessary for me to remind you that we are living in a changing world, and that both medicine and public health are playing a much more important role in the world than they did formerly. For example, it is now customary for public officials and people in general to talk about adequate medical care for every one; this is no longer merely the dream of the sociologist, but a real objective of the thinking public. I imagine that most of us, of the older generation, realize that this is a very high ideal, which has never yet been reached, even in the most advanced countries. It predicates further the utmost use of preventive as well as of curative medicine, otherwise the ideal could never be even remotely approached. The problem and the objective have recently been stated by Dean John Wyckoff of the New York University Medical School as follows: “Adequatemedical attention should be available to the public to the same extent as educational facffities.― You are no doubt familiar with the statements of Sir George Newman, Principal Medical Officer of Great Britain, who as an expert in medical education has coined the happy phrase that “themedical curriculum must be permeated with public health.― If this be the present attitude of educators, sociologists, and the leaders of the medical profession in the most advanced countries may we not ask and try to learn how it affects us in our special 1Pr@ident's address delivered at the thirtieth annual meeting of the Ameri can Society of Tropical Medicine, at San Antonio, Texas, November 15, 1934. -

Honduras Medical Mission Trip Information Packet Valle De Ángeles, Honduras Medical Mission Trip
April 21 – 28, 2020 Honduras Medical Mission Trip Information Packet Valle de Ángeles, Honduras Medical Mission Trip Trip Purpose Volunteers will provide primary care outpatient services and health education to several Honduran villages during five days of clinics. Location & Culture Honduras is bordered to the west by Guatemala, to the southwest by El Salvador, to the southeast by Nicaragua, to the south by the Pacific Ocean, and to the north by the Gulf of Honduras, a large inlet of the Caribbean Sea. The Honduran culture is very warm and friendly. When greeting, people often kiss each other’s cheeks and hugs are common as well. The culture is relaxed, where interpersonal relationships are valued more than punctuality keeping a timely schedule. Valle de Angeles is approximately 23 kilometers, a 45-minute bus ride, outside of the capital city of Tegucigalpa. It is one of the most pristine locations in the country and has a nearby national park, La Tigra, which is excellent for hiking and bird watching. We will be partnering with Hospital Adventista Valle de Angeles (HAVA) while in Honduras. HAVA is a fully functional private hospital complete with In-Patient Care, Emergency Department, Out-Patient Clinic, Operating Suite, and Physical Therapy. HAVA provides a tranquil and serene healing environment, far different from chaotic and crowded government-run hospitals located in Tegucigalpa. HAVA serves as the main medical facility for the 10,000-person community. Trip Cost and Due Dates The total trip cost is $1,300 Due Dates Payment Amount per Person Date Due Non-Refundable Deposit $200 Upon Application 50% of Trip Cost $550 January 10, 2020 100% of Trip Cost $550 March 13, 2020 TOTAL $1,300 Note: Airline tickets will be purchased once the 50% of the trip cost has been paid. -

GLOSSARY of TERMS WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT)
GLOSSARY OF TERMS WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT) WHO European Framework for Action on Integrated Health Services Delivery Glossary of terms WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT) Series editors Juan Tello, WHO Regional Office for Europe Erica Barbazza, University of Amsterdam Zhamin Yelgezekova, WHO European Centre for Primary Health Care Ioana Kruse, WHO European Centre for Primary Health Care Niek Klazinga, University of Amsterdam Dionne Kringos, University of Amsterdam WHO European Framework for Action on Integrated Health Services Delivery Abstract This glossary of terms aims to provide clarifying definitions related to the WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT). PHC-IMPACT sets out to support the monitoring and improvement of primary health care in the European Region and the measurement of progress towards the services delivery component of global universal health coverage targets. The framework underpinning PHC-IMPACT has been guided by the WHO European Framework for Integrated Health Services Delivery. This glossary of terms accompanies PHC-IMPACT’s Indicator Passports – a resource providing detailed information for the use of the full suite of indicators that make up the tool. Importantly, the definitions included here have relied as far as possible on existing international classifications including the International Classification for Health Accounts, International Standard Classification of Occupations and International Standard Classification of Education. Keywords HEALTH SERVICES PRIMARY HEALTH CARE HEALTH CARE SYSTEMS HEALTH POLICY EUROPE Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe UN City, Marmorvej 51 DK-2100 Copenhagen Ø Denmark Alternatively, complete an online request form for documentation, health information, or for permission to quote or translate, on the Regional Office website (http://www.euro.who.int/pubrequest).