Innovating to Fight Neglected Tropical Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lshtm-Prospectus.Pdf
Welcome The London School of Hygiene & Tropical Medicine (LSHTM) is widely recognised as a world-leading school of public and global health, working closely with partners in the UK and worldwide to address contemporary and future critical health challenges. Our commitment to improving Professor Peter Piot KCMG FMedSci health in the UK and worldwide is the lifeblood of LSHTM, and I am proud Peter Piot is the Director and Handa Contents of the work our staff, students and Professor of Global Health at the alumni do to address major health London School of Hygiene & Tropical inequalities and challenges. Medicine. Professor Piot was part of a team that co-discovered the Ebola INTRODUCTION 03 COURSES 16 We have a diverse and truly global community dedicated virus in 1976, and was a leading voice to quality cross-disciplinary research. LSHTM is involved during the recent Ebola outbreak in at every stage of the research pipeline, from basic science West Africa. He also instigated some Welcome 03 Clinical Trials 16 all the way through to evaluation of health interventions, of the first research programmes Why Choose LSHTM? 04 Epidemiology 18 providing a firm foundation of evidence for improving health. into HIV/AIDS, and was the founding Crucially, we take research out into the real world to make Executive Director of UNAIDS and Why Distance Learning? 05 Global Health Policy 20 a tangible difference in people’s lives. Under-Secretary-General of the LSHTM and University of London 06 Infectious Diseases 22 United Nations from 1995 until 2008. This year marks our School’s 120th anniversary and we Our Global Student Community 08 Public Health 24 are celebrating 120 years of health innovation at LSHTM. -

Travel Clinic Operations Guide Edition 5
Travel Clinic Operations Guide Edition 5 www.travax.com © 2016 Shoreland, Inc. All rights reserved. Travel Clinic Operations Guide – page 2 INTRODUCTION The Travel Clinic Operations Guide provides an overview of the resources and travel-specific information useful to those starting and maintaining an international travel medicine clinic or administering travel-related vaccines within the context of a medical practice. Additional considerations may apply to travel medicine clinics in other care delivery settings such as pharmacies, workplaces, and public health departments. Non-physician prescription of vaccines or travel-related medication is increasingly common, but varies widely by state or province, and local regulations need to be clearly understood. Materials have been designed to help standardize delivery of service and reduce administrative workload. This guide focuses on aspects of clinic operations that are unique to the practice of travel medicine. Resources, policies and procedures, and other guidelines applicable to general medical clinics can be found in a multitude of other publications and will not be provided here. ESTABLISHING A TRAVEL HEALTH CLINIC THE BASICS Because the concept of travel medicine is often new to travelers, it is important to take into consideration the unique aspects of establishing a travel medicine clinic. Location: A highly visible location on a main floor or centralized location will generate interest, prompt inquiries, and encourage drop- ins. A location within a well-care setting is also desirable. Parking: Because many travel medicine clinics offer evening or weekend appointments for busy travelers, parking should be both easily accessible and safe. Naming: Clinic names and signs should clearly indicate the unique services offered, such as travel immunizations. -

Ctropmed® Examination)
Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Health (CTropMed® Examination) Striving for Professional Excellence in Clinical Tropical Medicine and Travelers’ Health October 11–24, 2021 NEW IN 2021! The CTropMed® Examination is being offered globally through secure internet-based testing sites. Visit the list of testing centers here. Download Sample Exam Questions #TropMed21 Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Health (CTropMed® Examination) New in 2021! The CTropMed® Examination is being offered globally through secure internet-based testing sites. Visit the list of testing centers here. Table of Contents Fostering professional development in the fields of clinical tropical medicine and Requirements................. 3 travelers’ health is one of the Society’s highest priorities. To that end, ASTMH About the Examination .......... 5 developed the Certificate of Knowledge in Clinical Tropical Medicine and Travelers’ Applying for the Examination ..... 5 Health (CTropMed® Examination) as a means to distinguish individuals who have Testing Centers ............... 6 demonstrated advanced knowledge and experience in these fields. The Certificate Test Center Admission of Knowledge in Clinical Tropical Medicine and Travelers’ Health is conferred on Requirements................. 7 Prohibited Items............... 8 1) licensed healthcare professionals applying via Practice Pathway or those Cancellation and healthcare professionals (regardless of licensure) who have passed an ASTMH- Rescheduling Policy............ 9 accredited Diploma Course, 2) have direct experience in the fields of tropical Scoring ..................... 10 medicine and/or travelers’ health, and 3) have passed the ASTMH Examination in Examination Results............ 10 Clinical Tropical Medicine and Travelers’ Health. Special Circumstances.......... 10 ASTMH’s Committment to Inclusion and Respect .......... 11 To support this process, ASTMH accredits specific diploma courses that meet Examination Checklist ......... -
Programme Specification
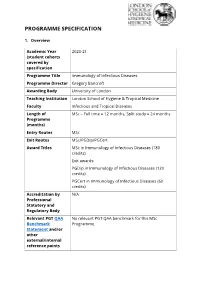
PROGRAMME SPECIFICATION 1. Overview Academic Year 2020-21 (student cohorts covered by specification Programme Title Immunology of Infectious Diseases Programme Director Gregory Bancroft Awarding Body University of London Teaching Institution London School of Hygiene & Tropical Medicine Faculty Infectious and Tropical Diseases Length of MSc – Full time = 12 months, Split study = 24 months Programme (months) Entry Routes MSc Exit Routes MSc/PGDip/PGCert Award Titles MSc in Immunology of Infectious Diseases (180 credits) Exit awards: PGDip in Immunology of Infectious Diseases (120 credits) PGCert in Immunology of Infectious Diseases (60 credits) Accreditation by N/A Professional Statutory and Regulatory Body Relevant PGT QAA No relevant PGT QAA benchmark for this MSc Benchmark Programme. Statement and/or other external/internal reference points Level of programme Masters (MSc) Level 7 within the Framework for Higher Education Qualifications (FHEQ) Total Credits CATS: 180 ECTS: 90 HECoS Code(s) 100345:100265:100948 (1:1:1) Mode of Delivery For 2020-21, the delivery of LSHTM teaching has been adjusted in response to the COVID-19 pandemic. For MSc Immunology of Infectious Diseases: Term 1 core module teaching will be delivered online only, with a combination of synchronous (live and interactive) and asynchronous (recordings, independent study, individual exercises, etc) activities. Terms 2 modules will be offered both online and also on-campus where possible. Some on-campus modules will use a blended approach of both face to face and online delivery if permitted under UK social distancing rules at that time. Term 3 will include on-campus pre- project lab training and access for students undertaking lab-based projects. -

Traveling Outside the United States? Get Vaccinated. Vaccines Protect You from Diseases Not Often Found in the United States
Traveling outside the United States? Get vaccinated. Vaccines protect you from diseases not often found in the United States. Plan ahead for safe, healthy travel. Get vaccinated 2 months before your trip. • Contact your healthcare provider or a travel clinic or pharmacy below. • Vaccines take time to fully protect you. • You may need multiple doses of vaccine, up to 4 weeks apart. Some travelers have special needs to consider. General information may not apply to your health situation or travel plans. You may need to schedule a consultation to determine how you should prepare for safe travel. Especially if you are: • Older than 65. • Traveling with a child younger than 2. • Pregnant. • Living with a medical condition. Online resources • Taking certain medications. Call before you go to Travel Health Online verify services and fees. tripprep.com Travel clinics Centers for Disease Control and Prevention Travelers’ Health Passport Health Virginia Mason Travel Clinic cdc.gov/travel 4301 S. Pine St., Suite 27 33501 First Way S. United States Department of State Tacoma, 98409 Federal Way, 98003 Travel Information (206) 414-2709 (206) 583-6585 travel.state.gov passporthealthusa.com virginiamason.org/travel-health World Health Organization International Travel and Health Pharmacies who.int/health-topics/travel-and-health International Society of Travel Medicine Rite Aid Fred Meyer istm.org Gig Harbor....(253) 851-6939 Bonney Lake ........(253) 891-7333 American Journal of Tropical Medicine Lakewood ....(253) 588-3666 Puyallup................(253) 840-8183 and Hygiene Tacoma .........(253) 474-0115 Sumner .................(253) 826-8433 ajtmh.org Tacoma .................(253) 475-6073 Safeway University Place ...(253) 460-4033 Milton ...........(253) 952-0390 Puyallup........(253) 841-1534 Tacoma .........(253) 566-9217. -

Glossary of Acronyms
Glossary of Acronyms Ab Antibody AFRO World Health Organization Regional Office for Africa ALB, Alb Albendazole AMRO World Health Organization Regional Office for the Americas (see PAHO) APOC World Health Organization African Programme for Onchocerciasis Control BICO Blantyre Institute for Community Opthamology BMGF Bill & Melinda Gates Foundation CAR Central African Republic CCA Circulating Cathodic Antigen CDC U.S. Centers for Disease Control & Prevention CRFilMT Center for Research on Filariasis and Other Tropical Diseases CWW Children Without Worms, The Task Force for Global Health DEC Diethylcarbamazine DOLF Death to Onchocerciasis and Lymphatic Filariasis Project, Washington University in St. Louis DRC Democratic Republic of Congo ELISA Enzyme-Linked Immunosorbent Assay EMRO World Health Organization Regional Office for the Eastern Mediterranean EURO World Health Organization Regional Office for Europe FHI, FHI360 Family Health International FPSU Filarial Programmes Support Unit, Liverpool School of Tropical Medicine (formerly CNTD) FTS Alere Filariasis Test Strip GSK GlaxoSmithKline GTMP Global Trachoma Mapping Project HDI Health and Development International HKI Helen Keller International IBB Institut de Bouisson Bertrand ICT Immunochromatography Test IRD Institut de Recherche pour le Développement ITI International Trachoma Initiative, The Task Force for Global Health IVM, Iver Ivermectin (Generic Name for Mectizan) JCU James Cook University JHU John Hopkins University KEMRI Kenya Medical Research Institute LF Lymphatic Filariasis -

The Educational Background for the Practice of Tropical Medicine
THE EDUCATIONAL BACKGROUND FOR THE PRAC-. TICE OF TROPICAL MEDICINE' FREDERICK F. RUSSELL From the International Health Board, Rockefeller Foundation, New York, N. Y. Received for publication November 14, 1934 My address wifi be brief, as I have really a very simple theme and it will not take much of your time for me to present it. It is unnecessary for me to remind you that we are living in a changing world, and that both medicine and public health are playing a much more important role in the world than they did formerly. For example, it is now customary for public officials and people in general to talk about adequate medical care for every one; this is no longer merely the dream of the sociologist, but a real objective of the thinking public. I imagine that most of us, of the older generation, realize that this is a very high ideal, which has never yet been reached, even in the most advanced countries. It predicates further the utmost use of preventive as well as of curative medicine, otherwise the ideal could never be even remotely approached. The problem and the objective have recently been stated by Dean John Wyckoff of the New York University Medical School as follows: “Adequatemedical attention should be available to the public to the same extent as educational facffities.― You are no doubt familiar with the statements of Sir George Newman, Principal Medical Officer of Great Britain, who as an expert in medical education has coined the happy phrase that “themedical curriculum must be permeated with public health.― If this be the present attitude of educators, sociologists, and the leaders of the medical profession in the most advanced countries may we not ask and try to learn how it affects us in our special 1Pr@ident's address delivered at the thirtieth annual meeting of the Ameri can Society of Tropical Medicine, at San Antonio, Texas, November 15, 1934. -

GLOSSARY of TERMS WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT)
GLOSSARY OF TERMS WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT) WHO European Framework for Action on Integrated Health Services Delivery Glossary of terms WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT) Series editors Juan Tello, WHO Regional Office for Europe Erica Barbazza, University of Amsterdam Zhamin Yelgezekova, WHO European Centre for Primary Health Care Ioana Kruse, WHO European Centre for Primary Health Care Niek Klazinga, University of Amsterdam Dionne Kringos, University of Amsterdam WHO European Framework for Action on Integrated Health Services Delivery Abstract This glossary of terms aims to provide clarifying definitions related to the WHO European Primary Health Care Impact, Performance and Capacity Tool (PHC-IMPACT). PHC-IMPACT sets out to support the monitoring and improvement of primary health care in the European Region and the measurement of progress towards the services delivery component of global universal health coverage targets. The framework underpinning PHC-IMPACT has been guided by the WHO European Framework for Integrated Health Services Delivery. This glossary of terms accompanies PHC-IMPACT’s Indicator Passports – a resource providing detailed information for the use of the full suite of indicators that make up the tool. Importantly, the definitions included here have relied as far as possible on existing international classifications including the International Classification for Health Accounts, International Standard Classification of Occupations and International Standard Classification of Education. Keywords HEALTH SERVICES PRIMARY HEALTH CARE HEALTH CARE SYSTEMS HEALTH POLICY EUROPE Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe UN City, Marmorvej 51 DK-2100 Copenhagen Ø Denmark Alternatively, complete an online request form for documentation, health information, or for permission to quote or translate, on the Regional Office website (http://www.euro.who.int/pubrequest). -

A Brief History of Global Health by M
An Introduction to Global Health and Global Health Ethics: A Brief History of Global Health By M. A. Palilonis Learning Objectives: 1. Identify population-level and individual-level health measures across the history of global health 2. Consider competing theories of justice in global health 3. Evaluate the arguments for and against wealthy nations to provide international aid A Brief History of Global Health In order to understand a broad concept, like global health, it is important to consider where it comes from. The history of global health will be told here in broad strokes and will follow two major trends that shaped global health organizations: population health through the control of infectious diseases and individual health through the delivery of healthcare. Each of the stages discussed here, tropical medicine, international health, the age of development and the rise of the NGO, overlap and converge. Overall, as the world became increasingly interconnected global health moved from the imperial concerns of “tropical medicine” to include more nations and other international organizations in the formations of international health policy. However, though the concept of global health changed greatly since its beginning, infection control and delivery of healthcare remained important core features of global health. Tropical Medicine This stage of global health is defined by the imperial system of colonization. In the 16th and 17th centuries European countries began to travel to new lands setting up the settlements that would eventually become the colonies of the 18th and 19th centuries. Countries such as Great Britain, France and Portugal founded colonies in places such as India, China and Africa, where settlers encountered new diseases and harsh climates.i Infectious diseases were devastating to both the native populations and to the European colonists. -

Travel Medicine What’S Involved? When to Refer?
Clinical Review Travel medicine What’s involved? When to refer? Brian Aw MD CCFP Suni Boraston MD MHSc David Botten MD CCFP Darin Cherniwchan MDCM CCFP FCFP Hyder Fazal MD FRCPC Timothy Kelton MD CCFP(EM) FCFP Michael Libman MD FRCPC Colin Saldanha MBBS Philip Scappatura MD Brian Stowe MBA Abstract Objective To defne the practice of travel medicine, provide the basics of a comprehensive pretravel consultation for international travelers, and assist in identifying patients who might require referral to travel medicine professionals. Sources of information Guidelines and recommendations on travel medicine and travel-related illnesses by national and international travel health authorities were reviewed. MEDLINE and EMBASE searches for related literature were also performed. Main message Travel medicine is a highly dynamic specialty that focuses on pretravel preventive care. A comprehensive risk assessment for each individual traveler is essential in order to accurately evaluate traveler-, itinerary-, and destination-specifc risks, and to advise on the most appropriate risk management interventions to promote EDITOR’S KEY POINTS health and prevent adverse health outcomes during travel. • Travel medicine is a multidisciplinary specialty Vaccinations might also be required and should be personalized that requires expertise in travel-related according to the individual traveler’s immunization history, travel illnesses, as well as up-to-date knowledge itinerary, and the amount of time available before departure. of the global epidemiology of infectious and noninfectious health risks, health regulations Conclusion A traveler’s health and safety depends on a and immunization requirements in various practitioner’s level of expertise in providing pretravel counseling countries, and the changing patterns of drug- and vaccinations, if required. -

An Approach to a Patient with Tropical Infection in the Intensive Care Unit Dilip R Karnad1 , Pravin Amin2
INVITED ARTICLE An Approach to a Patient with Tropical Infection in the Intensive Care Unit Dilip R Karnad1 , Pravin Amin2 Indian Journal of Critical Care Medicine (2021): 10.5005/jp-journals-10071-23867 INTRODUCTION 1Department of Critical Care, Jupiter Hospital, Thane, Maharashtra, Tropical diseases are often defined as diseases that are prevalent India 1 in, or unique to, tropical and subtropical regions of the world. 2Department of Critical Care, Bombay Hospital Institute of Medical Infections form a large proportion of the burden of tropical diseases. Sciences, Mumbai, Maharashtra, India These infections may sometimes require intensive care and tropical Corresponding Author: Dilip R Karnad, Department of Critical Care, infections account for about 20% of all intensive care unit (ICU) Jupiter Hospital, Thane, Maharashtra, India, Phone: +91 9892114992, 2 admissions in published data from Asia, South America, and Africa. e-mail: [email protected] In the INDICAPS study that included data from 4038 patients from How to cite this article: Karnad DR, Amin P. An Approach to a 124 ICUs in India, 231 (5.7%) patients had tropical infections and Patient with Tropical Infection in the Intensive Care Unit. Indian J Crit 3 50 (21.7%) of these died. In another study, Parikh et al. found that Care Med 2021;25(Suppl 2):S118–S121. almost 24% of 1116 patients admitted to the ICU of a public hospital Source of support: Nil 4 in Mumbai were for tropical infections. Yeolekar et al. mention that Conflict of interest: None in the monsoon months, almost 80% of ICU beds in public hospitals may be occupied by patients with tropical infections.5 Tropical infections are common in the geographic regions of the world that are close to the equator because they have references regarding diseases that can be acquired by travelers a warmer climate with less seasonal variation in temperatures, after visiting specific geographic areas. -

Prevention and Control of Tropical Diseases in the 21St Century: Back to the Field
Prevention and Control of Tropical Diseases in the 21st Century: Back to the Field Duane J. Gubler, Sc.D. Director Division of Vector-Borne Infectious Diseases National Center for Infectious Diseases Centers for Disease Control and Prevention Public Health Service U.S. Department of Health and Human Services PO Box 2087 Fort Collins, CO 80522 H:\ASTMH\ASSOCIATION DOCUMENTS\ANNUAL MEETING\2000 - HOUSTON\PRESIDENTIAL ADDRESS.DOC Introduction First let me say what a great honor it is for me to be the President of the American Society of Tropical Medicine and Hygiene. This represents the pinnacle of my career, and I want to thank all of my colleagues and collaborators who, over the past 35 years, have provided me the many opportunities to work in this field. Their constant encouragement and support allowed me to develop and grow. Most important, I want to thank my wife, Bobbie, for the support and encouragement she has given me over the years; she must share equally in any success I have had. She was always supportive and encouraged me to go where the action was even though it meant my being away in the field for weeks at a time without her knowing where I was or what I was doing; remember the days before satellite and cell telephones? But it was her support and encouragement that allowed me to live and work in the field, which is so important to tropical medicine research. And, I want her to know how much I appreciate her dedication to my career. H:\ASTMH\ASSOCIATION DOCUMENTS\ANNUAL MEETING\2000 - HOUSTON\PRESIDENTIAL ADDRESS.DOC Page 2 of 28 The understanding and support I received from my immediate family allowed me to spend most of my career in the field.