CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Chennai Comprehensive Transportation Study (CCTS)
ACKNOWLEDGEMENT The consultants are grateful to Tmt. Susan Mathew, I.A.S., Addl. Chief Secretary to Govt. & Vice-Chairperson, CMDA and Thiru Dayanand Kataria, I.A.S., Member - Secretary, CMDA for the valuable support and encouragement extended to the Study. Our thanks are also due to the former Vice-Chairman, Thiru T.R. Srinivasan, I.A.S., (Retd.) and former Member-Secretary Thiru Md. Nasimuddin, I.A.S. for having given an opportunity to undertake the Chennai Comprehensive Transportation Study. The consultants also thank Thiru.Vikram Kapur, I.A.S. for the guidance and encouragement given in taking the Study forward. We place our record of sincere gratitude to the Project Management Unit of TNUDP-III in CMDA, comprising Thiru K. Kumar, Chief Planner, Thiru M. Sivashanmugam, Senior Planner, & Tmt. R. Meena, Assistant Planner for their unstinted and valuable contribution throughout the assignment. We thank Thiru C. Palanivelu, Member-Chief Planner for the guidance and support extended. The comments and suggestions of the World Bank on the stage reports are duly acknowledged. The consultants are thankful to the Steering Committee comprising the Secretaries to Govt., and Heads of Departments concerned with urban transport, chaired by Vice- Chairperson, CMDA and the Technical Committee chaired by the Chief Planner, CMDA and represented by Department of Highways, Southern Railways, Metropolitan Transport Corporation, Chennai Municipal Corporation, Chennai Port Trust, Chennai Traffic Police, Chennai Sub-urban Police, Commissionerate of Municipal Administration, IIT-Madras and the representatives of NGOs. The consultants place on record the support and cooperation extended by the officers and staff of CMDA and various project implementing organizations and the residents of Chennai, without whom the study would not have been successful. -
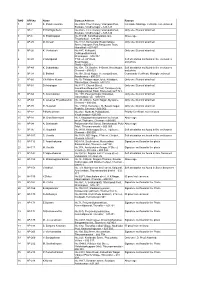
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Arakkonam Section on 14Th August 2021
दक्षिण रेलवे/Southern Railway चेन्नै मंडल/Chennai Division No.PUB/MAS/2021/08/13 Date:13.08.2021 प्रेसववज्ञप्ति /PRESS RELEASE CHANGES IN PATTERN OF SUBURBAN TRAIN SERVICES According, priority to Passenger Safety and Safety of train operations, as a part of ongoing Engineering works, Line Block/Power Block is permitted in Chennai Central - Arakkonam section on UP Slow line between Villivakkam and Vyasarpadi Jiva stations on 14th August 2021 from 11:05 hrs to 13:05 hrs (2 Hours). Consequently, the following are the changes in pattern of suburban train services. SUBURBAN TRAIN SERVICES FULLY CANCELLED 1.Train No. VBAD3, Velachery – Avadi Workmen Special leaving Velachery at 10:15 hrsis FULLY CANCELLED. 2.Train No. ADM14, Avadi–Moore Market Complex Workmen Special leaving Avadi at 11:00 hrsis FULLY CANCELLED. 3.Train No. ADBV12, Avadi – VelacheryWorkmen Special leaving Avadi at 12:10 hrs is FULLY CANCELLED. PARTIAL CANCELLATION OF SUBURBAN TRAIN SERVICES 1.Train No. MK13, Moore Market Complex – Kadambathur Workmen Special leaving Moore Market Complex at 12:10 hrs is PARTIALLY CANCELLEDbetween Moore Market Complex and Avadi. CHANGES IN ORIGINATING STATION OF SUBURBAN TRAIN SERVICES 1.Train No. MS11, Moore Market Complex – Pattabiram Military Siding ‘E’ Depot Workmen Special leaving Moore Market Complex at 12:20 hrswill originate from Chennai Beachat 12:20 hrs. 2.Train No. MT25, Moore Market Complex – Tiruvallur Workmen Special leaving Moore Market Complex at 13:00 hrs will originate from Chennai Beach at 13:00 hrs. 3.Train No. MS13, Moore Market Complex – Pattabiram Military Siding ‘E’ Depot Workmen Special leaving Moore Market Complex at 13:15 hrs will originate from Chennai Beach at 13:15 hrs. -

33L Bus Time Schedule & Line Route
33L bus time schedule & line map 33L High Court - Kodungaiyur(Parvathi Nagar) View In Website Mode The 33L bus line (High Court - Kodungaiyur(Parvathi Nagar)) has 4 routes. For regular weekdays, their operation hours are: (1) High Court: 5:16 AM - 7:00 PM (2) Kodungaiyur Parvathi Nagar: 4:56 AM - 12:35 PM (3) Kodungaiyur(Parvathi Nagar): 6:03 AM - 8:05 PM (4) M.K.B.Nagar East: 12:12 PM - 8:52 PM Use the Moovit App to ƒnd the closest 33L bus station near you and ƒnd out when is the next 33L bus arriving. Direction: High Court 33L bus Time Schedule 34 stops High Court Route Timetable: VIEW LINE SCHEDULE Sunday 5:16 AM - 7:00 PM Monday 5:16 AM - 7:00 PM Kodungaiyur (Parvathi Nagar) Tuesday 5:16 AM - 7:00 PM Gandhi Nagar Wednesday 5:16 AM - 7:00 PM Gandhi Silai Thursday 5:16 AM - 7:00 PM Sidco Friday 5:16 AM - 7:00 PM Sidco Saturday 5:16 AM - 7:00 PM Electricity Board O∆ce T.N.E.B. O∆ce 33L bus Info Direction: High Court M.K.B.Nagar Stops: 34 MKB Nagar West Ave, Chennai Trip Duration: 29 min Line Summary: Kodungaiyur (Parvathi Nagar), M.K.B. Nagar Gandhi Nagar, Gandhi Silai, Sidco, Sidco, Electricity Board O∆ce, T.N.E.B. O∆ce, M.K.B.Nagar, M.K.B. Vyasarpadi Depot Nagar, Vyasarpadi Depot, Ambedkar College, Erikarai, Vyasarpadi Market, Vyasarpadi, Ramalinga Ambedkar College Kovil, Basin Bridge, Moolakkothanam, Molakoththalam, V Nagar, V Nagar, Stanley Hospital, Erikarai Bharathi Womens College, Thambu Chetty, Clive Battery, Collector O∆ce, Chennai Beach, Kall Vyasarpadi Market Mandapam, Chennai Beach Station, Ranga Vilas, Parrys Corner, Parrys -
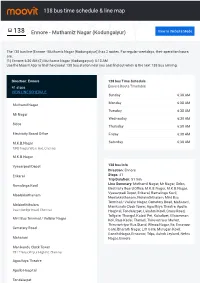
138 Bus Time Schedule & Line Route
138 bus time schedule & line map 138 Ennore - Muthamiz Nagar (Kodungaiyur) View In Website Mode The 138 bus line (Ennore - Muthamiz Nagar (Kodungaiyur)) has 2 routes. For regular weekdays, their operation hours are: (1) Ennore: 6:30 AM (2) Muthamiz Nagar (Kodungaiyur): 5:13 AM Use the Moovit App to ƒnd the closest 138 bus station near you and ƒnd out when is the next 138 bus arriving. Direction: Ennore 138 bus Time Schedule 41 stops Ennore Route Timetable: VIEW LINE SCHEDULE Sunday 6:30 AM Monday 6:30 AM Muthamil Nagar Tuesday 6:30 AM Mr Nagar Wednesday 6:30 AM Sidco Thursday 6:30 AM Electricity Board O∆ce Friday 6:30 AM M.K.B.Nagar Saturday 6:30 AM MKB Nagar West Ave, Chennai M.K.B.Nagar Vyasarpadi Depot 138 bus Info Direction: Ennore Erikarai Stops: 41 Trip Duration: 51 min Ramalinga Kovil Line Summary: Muthamil Nagar, Mr Nagar, Sidco, Electricity Board O∆ce, M.K.B.Nagar, M.K.B.Nagar, Vyasarpadi Depot, Erikarai, Ramalinga Kovil, Moolakkothanam Moolakkothanam, Molakoththalam, Mint Bus Terminal / Vallalar Nagar, Cemetery Road, Maharani, Molakoththalam Manikundu Clock Tower, Agasthiya Theatre, Apollo Basin Bridge Road, Chennai Hospital, Tondaiarpet, Lakshmi Kovil, Cross Road, Tollgate, Thangal, Kaladi Pet, Kaladipet, Ellaiamman Mint Bus Terminal / Vallalar Nagar Koil, Raja Kadai, Theradi, Thiruvotriyur Market, Thiruvortriyur Bus Stand, Wimco Nagar, Itc, Ernavoor Cemetery Road Gate, Bharath Nagar, Lift Gate, Murugan Kovil, Gandhi Nagar, Ernavoor, Tdps, Ashok Leyland, Nehru Maharani Nagar, Ennore Manikundu Clock Tower 781 Thiruvottriyur -

CHAITANYA Tep Hen Son Rd
Friends BSNL Football Club Ground Vyasarpadi ain Rd. Jeeva erambur M M P Kannigapuram a in Ground R o a d S CHAITANYA tep hen son Rd. Perambur . Bus Depot d D R eca ter R s d e d De d . m g e B&C Mills a a ll o d w o i o s r R R R d B s s . Choolai n k i k c s o a a r o B r C a B r u Strahans Rd. b m a Map not to Scale r e P Divine living, inspired by tradition North Town Estates Pvt. Limited An Arihant - Unitech - PVP Initiative Site Office: Buckingham and Carnatic Gardens, Door No.- 4, 5, 6 and 7, Stephenson Road, Perambur, Chennai - 600012. Ph.: +91 44 28363266, 28364577 Mobile: +91 7299091111 Website: www.unitechgroup.com | www.arihantfoundations.com An Arihant - Unitech - PVP Initiative North, ruled by lord of wealth 'Kubera' in hindu mythology, is one of the four cardinal directions considered CHAITANYA as most auspicious; symbolic of wealth, business, knowledge Escape the world and usher into new phase of Chaitanya. and happiness in an A vivacious planned development where divine living is inspired by tradition. enlightened environment. Welcome to Chaitanya at North Town – a premier residential development & experience a living of fulfillment, detachment and equanimity in your daily life . Nestled in the North-East corner of North Town, Chaitanya offers homes in 1, 2 & 3 bedroom apartments to suit individual needs. Experience a retreat to enjoy absolute calm and serenity, a special place to be at peace with one’s inner self. -

District Statistical Hand Book Chennai District 2016-2017
Government of Tamil Nadu Department of Economics and Statistics DISTRICT STATISTICAL HAND BOOK CHENNAI DISTRICT 2016-2017 Chennai Airport Chennai Ennoor Horbour INDEX PAGE NO “A VIEW ON ORGIN OF CHENNAI DISTRICT 1 - 31 STATISTICAL HANDBOOK IN TABULAR FORM 32- 114 STATISTICAL TABLES CONTENTS 1. AREA AND POPULATION 1.1 Area, Population, Literate, SCs and STs- Sex wise by Blocks and Municipalities 32 1.2 Population by Broad Industrial categories of Workers. 33 1.3 Population by Religion 34 1.4 Population by Age Groups 34 1.5 Population of the District-Decennial Growth 35 1.6 Salient features of 1991 Census – Block and Municipality wise. 35 2. CLIMATE AND RAINFALL 2.1 Monthly Rainfall Data . 36 2.2 Seasonwise Rainfall 37 2.3 Time Series Date of Rainfall by seasons 38 2.4 Monthly Rainfall from April 2015 to March 2016 39 3. AGRICULTURE - Not Applicable for Chennai District 3.1 Soil Classification (with illustration by map) 3.2 Land Utilisation 3.3 Area and Production of Crops 3.4 Agricultural Machinery and Implements 3.5 Number and Area of Operational Holdings 3.6 Consumption of Chemical Fertilisers and Pesticides 3.7 Regulated Markets 3.8 Crop Insurance Scheme 3.9 Sericulture i 4. IRRIGATION - Not Applicable for Chennai District 4.1 Sources of Water Supply with Command Area – Blockwise. 4.2 Actual Area Irrigated (Net and Gross) by sources. 4.3 Area Irrigated by Crops. 4.4 Details of Dams, Tanks, Wells and Borewells. 5. ANIMAL HUSBANDRY 5.1 Livestock Population 40 5.2 Veterinary Institutions and Animals treated – Blockwise. -

Dr.T.Prabhu 2016
Curriculum Vitae Dr.T.Prabhu 2016 Assistant Professor, Department of English, Dr.Ambedkar Govt. Arts College Vyasarpadi Chennai 600 039 Email- [email protected] Mobile-09994219296 Dr.T.Prabhu Assistant Professor, Department of English, Dr.Ambedkar Govt. Arts College Vyasarpadi Chennai 600 039 Email- [email protected] Mobile-09994219296 Years of Experience : 5 years DOB: 22/01/1984 Additional Responsibilities held Nodal Officer - AISHE at SS Govt. Arts College, Tiruttani : Coordinator - RUSA Coordinator - College Website Member - College NAAC Committee Research Experience Area of Research : Indigenous Studies Course Title of the Research Works Research Guide Ph.D „Memory‟, „History‟ and „Identity‟: Restoration of Native (2009-13) Sensibility in the Indigenous Epistemology Dr.S.Armstrong Prof. and Head M.Phil Restoration of Indigenous Identity in the Late 19th and Early Dept. of English (2007-08) 20th Century Tamilakam Univ. of Madras Chennai M.A Louis Dumont‟s Dichotomy between Purity and Pollution in Dr.G.M.James (2005-07) Select Dalit Novels Head, Dept. of English Loyola College Chennai Research Projects S.No Title of the Project Funding Agency Period Amount Sanctioned 1. Bhārathakathai as Oral History: Mahābāratha UGC 2014-16 Rs.2,75,000 Performances in Tiruvallur District of Tamil (Minor Research Nadu Project) 2. Elements of Oral History in the Mahabaratha TANSCHE 2014-15 Rs.1,00,000 Therukoothu: Folk Performances during the (Minor Research Draupathi Amman Festival in Tiruttani Project) Publications 1. “Proxemics: Some Challenges and Strategies in Non-verbal Communication”. IUP Journal of Soft skills Vol. IV No. 3, September 2010. ISSN 0973-8479. -
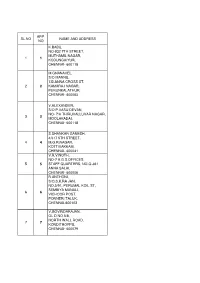
Sl.No App. No Name and Address 1 1 K.Babu, No-932
APP. SL.NO NAME AND ADDRESS NO K.BABU, NO-932 7TH STREET, MUTHAMIL NAGAR, 1 1 KODUNGAIYUR, CHENNAI- 600118 M.GNANAVEL, S/O MANNU, 7.B.ANNA CROSS ST, 2 2 KAMARAJ NAGAR, PERUNKALATHUR, CHENNAI- 600063 V.ALEXANDER, S/O P.VASU DEVAN, NO- 7/A THIRUVALLUVAR NAGAR, 3 3 MOOLAKADAI, CHENNAI- 600118 S.SHANKAR GANESH, 4/317 5TH STREET, 4 4 M.G.R.NAGAR, KOTTIVAKKAM, CHENNAI- 600041 V.R.VINOTH, NO-7 A.G.S.OFFICES, 5 5 STAFF QUARTERS, NO-Q-361 ANNA SALAI, CHENNAI- 600006 R.ANTHONI, S/O.S.K.RA JAN, NO.5/91, PERUMAL KOIL ST, SEMBIYA MANALI, 6 6 VICHOOR POST, PONNERI TALUK, CHENNAI-600103 V.GOVINDARAJAN, OL D NO.5/8, NORTH WALL ROAD, 7 7 KONDITHOPPU, CHENNAI- 600079 G.SIVAKUMAR, NO- 39/12 GANGAIAMMAN KOIL ST, 8 8 LAKSHMIPURAM, THIRUVANMIYUR CHENNAI- 600041 D.MOHAN, N0.22,KARUNANITHI ST, 9 9 KODUNGAYUR, CHENNAI- 600118 G.KARTHIKEYAN, 56,III RD BLOCK, HOUSING BOARD, 10 10 SATHYAMURTY NAGAR, VYASARPADI, CHENNAI-600039 C.SRINIVASAN, 1, 88TH SETREET, 11 11 ASHOK NAGAR, CHENNAI-600083 S,SIVASUBRAMANI, NO.137,5-BLOCK, 4THFLOOR, 12 12 HOUSING BOARD, PERIYAR NAGAR, PULIANTHOPE, CHENNAI- 600012 N.SATHISH, 13 13 NO.27, RADAS NAGAR, CHENNAI- 600021 D.SHANMUGAM, 69/37, ANGALAMMAN KOIL ST, 14 14 GOVINDAPURAM, CHENNAI- 600012 V. MUNIRAJ, 59, SOLAIAMMAN ST,, KODUNGAIYUR, 15 15 CHENNAI- 600118 C.KARNAN, N.NO.24,ARULAYAMMANPET, 16 16 GUINDY CHENNAI-600032 K.KARTHICK, NO.9,PER IYA PALAYATHAMAN KOIL , 17 17 7TH ST, MOOLAKOTHALAM, CHENNAI- 600021 DILLIBABU M, NO.5, ELUMALAI ST, 18 18 SAIDAPET, CHENNAI- 600015 R.MURUGAN, NEW NO.172,OLD NO.203, DOSS NAGAR, 19 19 5TH STREET, -

School-Phone-Nos
SCHL_NAME SCHOOL ADDRESS PHONE HM MOBILE 89, 2ND MAIN ROAD, JAGANATHAM NAGAR GOVT HS VILLIWAKKAM CH 49 SOUTH, VILLIVAKKAM, CHENNAI 26170816V.MURUGESAN 9381234553 GOVT HSS GKMCOLONY CHENNAI 82 GKM COLONY, CHENNAI 25509881T RAJAGOPAL 9841052090 GOVT HS BV COLONY VYASARPADI BV COLONY , VYASARPADI, CHENNAI 25513210 JEBAKUMARI BREMAHGNANAKILI9840266547 5TH BLOCK, KKD NAGAR, KODUNGAIYUR, GOVT HS KODUNGAIYUR CH 118 CHENNAI 25544994 R.GOPALAKRISHNAN 9841836254 GOVT HSS MKB NAGAR VYASARPADI MKB NAGAR, VYASARPADI,CHENNAI 26733710 K SEKAR 9444766336 GOVT GIRLS HSS VILLIVAKKAM SIDCONAGAR, VILLIVAKKAM, CHENNAI 26172919R BOMMAKKAL 9444312767 CHENNAI HS STRAHANS ROAD CH 12 4 STRAHANS ROAD, PATTALAM,CHENNAI 26625538 K NAGALAKSHMI 9941816876 CHENNAI HSS BUNDERGARDEN CH-11 NO 38 BUNDER GARDEN ST, CHENNAI 25583683M MURUGAIYAN 9840493327 35,CHINNA BABU STREET, NAMMALWARPET, CHENNAI HS NAMMALWARPET CH- 26472750MUNIKUMARI 9840956058 CHENNAI HSS AYANAVARAM CH 23 NO 1 PALAVOYAL STREET, AYANAVARAM 26742672C KALAI SELVI 7305860299 CHENNAI HS N P KOIL ST CH-1 N.P.KOIL STREET, CHENNAI 25588123SHEEBA .S. 8148877295 CHENNAI G HS CHENNAI 12 26, COOKS ROAD, CHENNAI - 12 26622375 T.PADMAVATHI 9840518909 53, MARKET STREET, PERAMBUR, CHENNAI - CHENNAI GHSS MARKET ST CHENNAI 11 26703477ALAMELU 9176322667 CHENNAI HS WALTAXRD CHENNAI 79 61, WALLTAX ROAD, CHENNAI 25296245R.RAJAKUMARI 9789044906 CHENNAI HS AGARAM CH 82 SOMAIAH RAJA STREET, AGARAM, CHENNAI 26703198S.CHANDAR 9444954941 29-A, GANGADEESWARAR KOIL ST, PURASAI, CHENNAI B HSS GK ST CHENNAI 84 CH-7 26403388 SHEILA ROSE -
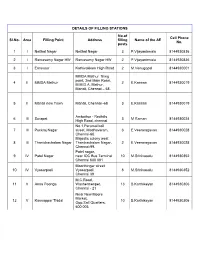
DETAILS of FILLING STATIONS Sl.No. Area Filling Point Address
DETAILS OF FILLING STATIONS No.of Cell Phone Sl.No. Area Filling Point Address filling Name of the AE No. posts 1 I Neithal Nagar Neithal Nagar 3 P.Vijayanirmala 8144930836 2 I Ramasamy Nagar HW Ramasamy Nagar HW 2 P.Vijayanirmala 8144930836 3 I Ernavoor Kathivakkam High Road 2 M.Venugopal 8144930001 MMDA Mathur filling point, 2nd Main Road, 4 II MMDA Mathur 2 E.Kannan 8144930019 M.M.D.A, Mathur, Manali, Chennai – 68. 5 II Manali new Town Manali, Chennai -68 3 E.Kannan 8144930019 Ambathur - Redhills 6 III Surapet 3 M Raman 8144930024 High Road, chennai No.1,Perumal koil 7 III Puckraj Nagar street, Madhavaram, 3 E.Veeraragavan 8144930028 Chennai-60. Majestic colony west 8 III Thanickachalam Nagar Thanikachalam Nagar, 2 E.Veeraragavan 8144930028 Chennai-99. Patel nagar, 9 IV Patel Nagar near IOC Bus Terminal 10 M.Srinivasalu 8144930352 Chennai 600 081 Moorthingar street 10 IV Vyasarpadi Vyasarpadi 8 M.Srinivasalu 8144930352 Chennai 39 M.C.Road, 11 V Anna Poonga Washermenpet, 13 B.Karthikeyan 8144930306 Chennai - 21 Near New Moore Market, 12 V Kannappar Thidal 10 B.Karthikeyan 8144930306 Opp.Salt Quarters, 600 003 DETAILS OF FILLING STATIONS No.of Cell Phone Sl.No. Area Filling Point Address filling Name of the AE No. posts Kolathur Water Distribution Station, 200 Kolathur Feet Road, Jawaharlal 13 VI 14 Richard Thomas 8144930689 Nehru Salai, (Near Rettai Eri jn), Kolathur, Chennai-99 Goodwill Apartment 14 VII Mogappair West Road, Mogappair West, 7 C.R.Sweetlin Jaba 8144930354 Chennai-600 037. 100 feet Road, near 15 VII Kolathur 1 V.Madhusudhanan 8144930084 Rettaieari Junction Kallikuppam Head Works, 16 VII Kallikuppam Pillaiyar Koil Street, 2 Dharmalingam 8144930085 Kallikuppam, Chennai New Avadi road, 17 VIII K.P.S. -

Session – 3 River and Drainage System In
SESSION – 3 RIVER AND DRAINAGE SYSTEM IN CMA Session – III Waterways in Chennai Thiru T.Kanthimathinathan, Eexecutive Engineer, PWD & Nodal Officer, Cooum Sub Basin Restoration & Management CHENNAI METROPOLITAN AREA • Chennai Metropolitan Area (CMA) covers 1189 Sq. Km. present population about 75 Lakhs projected to 98 Lakhs in 2011 • Chennai City covers 176 Sq. Km. having Terrain slope varying from 1 : 5000 to 1 : 10,000 • The City is drained by 2 rivers besides a number of major & minor drains through Buckingham Canal into Sea via Ennore Creek, Cooum mouth, Adyar mouth and Kovalam Creek. • Major Flood Events in Chennai City experienced during 1943, 1976, 1985,1996 & 2005 177 RIVERS AND DRAINAGE SYSTEM OF CHENNAI METROPOLITAN AREA Km. Orgin in Km. in Km. System in Sq.Km. 2005 in C/s. 2005 in C/s. Capacity in Capacity C/s Bed width in M. in Bed width River / Drainage Anticipated flood flood Anticipated discharge/ Presnet discharge/ Presnet Length in CMA in in CMA Length Flood discharge in Flood discharge with Bay of Bengal Total Length in Km. in Length Total Length in City Limits Limits in City Length Total Catchment Area Area Catchment Total Location of confluence confluence Location of RIVERS Krishnapuram (AP) for nagri am / 150 Kaveripakkam 125000/ Kosasthalaiyar Ennore Creek 136 16 3757 to 90000 (Vellore District) 110000 250 for Kosasthalaiyar arm Cooum Tank (Thiruvallur Cooum District) 40 to 22000/ Cooum Mouth near 72 18 40 400 21500 Kesavaram for 120 19500 Napier Bridge diversion from Kosasthalaiyar 10.50 Adanur Tank near 60000/