Locomotion of the Red-Shanked Douc Langur (Pygathrix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hunting, Law Enforcement, and African Primate Conservation
Research Note Hunting, Law Enforcement, and African Primate Conservation PAUL K. N’GORAN,∗†‡§ CHRISTOPHE BOESCH,∗†‡ ROGER MUNDRY,∗ ELIEZER K. N’GORAN,∗∗ ILKA HERBINGER,‡ FABRICE A. YAPI,†† AND HJALMAR S. KUHL¨ ∗‡‡ ∗Department of Primatology, Max Planck Institute for Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, Germany †Centre Suisse de Recherches Scientifiques en Cote-d’Ivoire,ˆ 01 BP 1303 Abidjan 01, Coteˆ d’Ivoire ‡Wild Chimpanzee Foundation s/c CSRS 01 BP 1303 Abidjan 01, Coteˆ d’Ivoire §UFR Sciences de la Nature, Universite´ d’Abobo-Adjame,´ 02 BP 801 Abidjan 02, Coteˆ d’Ivoire ∗∗UFR Biosciences, Universite´ de Cocody, 01 BP V34 Abidjan 01, Coteˆ d’Ivoire ††Office Ivoirien des Parcs et Reserves,´ Direction de Zone Sud-Ouest, BP 1342 Soubre,´ Coteˆ d’Ivoire Abstract: Primates are regularly hunted for bushmeat in tropical forests, and systematic ecological monitor- ing can help determine the effect hunting has on these and other hunted species. Monitoring can also be used to inform law enforcement and managers of where hunting is concentrated. We evaluated the effects of law enforcement informed by monitoring data on density and spatial distribution of 8 monkey species in Ta¨ıNa- tional Park, Coteˆ d’Ivoire. We conducted intensive surveys of monkeys and looked for signs of human activity throughout the park. We also gathered information on the activities of law-enforcement personnel related to hunting and evaluated the relative effects of hunting, forest cover and proximity to rivers, and conservation effort on primate distribution and density. The effects of hunting on monkeys varied among species. Red colobus monkeys (Procolobus badius) were most affected and Campbell’s monkeys (Cercopithecus campbelli) were least affected by hunting. -

Title SOS : Save Our Swamps for Peat's Sake Author(S) Hon, Jason Citation
Title SOS : save our swamps for peat's sake Author(s) Hon, Jason SANSAI : An Environmental Journal for the Global Citation Community (2011), 5: 51-65 Issue Date 2011-04-12 URL http://hdl.handle.net/2433/143608 Right Type Journal Article Textversion publisher Kyoto University SOS: save our swamps for peatʼs sake JASON HON Abstract The Malaysian governmentʼs scheme for the agricultural intensification of oil palm production is putting increasing pressure on lowland areas dominated by peat swamp forests.This paper focuses on the peat swamp forests of Sarawak, home to 64 per cent of the peat swamp forests in Malaysia and earmarked under the Malaysian governmentʼs Third National Agriculture Policy (1998-2010) for the development and intensification of the oil palm industry.Sarawakʼs tropical peat swamp forests form a unique ecosystem, where rare plant and animal species, such as the alan tree and the red-banded langur, can be found.They also play a vital role in maintaining the carbon balance, storing up to 10 times more carbon per hectare than other tropical forests. Draining these forests for agricultural purposes endangers the unique species of flora and fauna that live in them and increases the likelihood of uncontrollable peat fires, which emit lethal smoke that can pose a huge environmental risk to the health of humans and wildlife.This paper calls for a radical reassessment of current agricultural policies by the Malaysian government and highlights the need for concerted effort to protect the fragile ecosystems of Sarawakʼs endangered -

Development and the Evolvability of Human Limbs
Development and the evolvability of human limbs Nathan M. Younga,1, Günter P. Wagnerb, and Benedikt Hallgrímssonc aDepartment of Orthopaedic Surgery, University of California, San Francisco, CA 94110; bDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06405; and cDepartment of Cell Biology and Anatomy, University of Calgary, Calgary, AB, Canada T2N4N1 Edited* by David Pilbeam, Harvard University, Cambridge, MA, and approved December 29, 2009 (received for review October 14, 2009) The long legs and short arms of humans are distinctive for a fore- and hindlimb modules was reduced, leading to a higher primate, the result of selection acting in opposite directions on degree of variational independence of limb size and shape. Our each limb at different points in our evolutionary history. This model predicts that selection for independent function of the mosaic pattern challenges our understanding of the relationship limbs should lead to alterations to limb covariational structure. of development and evolvability because limbs are serially homol- Specifically, humans and apes would be predicted to exhibit ogous and genetic correlations should act as a significant con- decreased phenotypic correlations between fore- and hindlimb straint on their independent evolution. Here we test a compared to quadrupeds. If decreases in integration between developmental model of limb covariation in anthropoid primates fore- and hindlimb are a product of selection for more inde- and demonstrate that both humans and apes exhibit significantly pendent function of limbs, then the timing of any change in reduced integration between limbs when compared to quadrupe- integration has implications for the reconstruction of ancestral dal monkeys. -
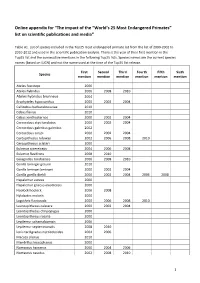
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Coexistence of Confamilial, Folivorous Indriids, Propithecus Diadema And
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Spring 5-15-2017 Coexistence of Confamilial, Folivorous Indriids, Propithecus diadema and Indri indri, at Betampona Strict Nature Reserve, Madagascar Lana Kerker Oliver Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/art_sci_etds Part of the Biodiversity Commons, Biological and Physical Anthropology Commons, Natural Resources and Conservation Commons, and the Natural Resources Management and Policy Commons Recommended Citation Oliver, Lana Kerker, "Coexistence of Confamilial, Folivorous Indriids, Propithecus diadema and Indri indri, at Betampona Strict Nature Reserve, Madagascar" (2017). Arts & Sciences Electronic Theses and Dissertations. 1134. https://openscholarship.wustl.edu/art_sci_etds/1134 This Dissertation is brought to you for free and open access by the Arts & Sciences at Washington University Open Scholarship. It has been accepted for inclusion in Arts & Sciences Electronic Theses and Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Department of Anthropology Dissertation Examination Committee Crickette Sanz, Chair Kari Allen Benjamin Z. Freed Jane Phillips-Conroy David Strait Mrinalini Watsa Coexistence of Confamilial, Folivorous Indriids, Propithecus diadema and Indri indri, at Betampona Strict -

A Note on Responses of Juvenile Javan Lutungs (Trachypithecus Auratus Mauritius) Against Attempted Predation by Crested Goshawks (Accipter Trivirgatus)
人と自然 Humans and Nature 25: 105−110 (2014) Report A note on responses of juvenile Javan lutungs (Trachypithecus auratus mauritius) against attempted predation by crested goshawks (Accipter trivirgatus) Yamato TSUJI 1*, Hiroyoshi HIGUCHI 2 and Bambang SURYOBROTO 3 1 Primate Research Institute, Kyoto University, Inuyama, Japan 2 Graduate School of Media and Governance, Keio University, Fujisawa, Japan 3 Bogor Agricultural University, Java West, Indonesia Abstract On October 31st, 2013, we unexpectedly observed attempted predation of two juvenile Javan lutungs (Trachypithecus auratus mauritius) by adult crested goshawks (Accipter trivirgatus), in Pangandaran Nature Reserve, West Java, Indonesia. The goshawks flew over a group of feeding lutungs, attempting attacks only on juvenile lutungs from the rear, while emitting tweeting calls. The attacks involved two different birds (probably a pair) in turn from different directions. The attacks by the goshawks were repeated six times during our observation period (between 13:51 and 13:58), but none of the attempts was successful. During the attacks, no other lutungs in the group showed any anti-predator behavior (such as emitting alarm calls or escaping) against the goshawks perhaps because their body weight (6-10 kg) was much larger than that of goshawk (0.4-0.6 kg). To our knowledge, this is the first detailed report of hunting-related behavior to primates by this raptor species. Key words: Accipter trivirgatus, crested goshawk, Javan lutung, Pangandaran, predation, Trachypithecus auratus mauritius Introduction primates has been conducted for over 50 years. However, with the exception of Philippine eagles Predation is considered to be a principal selective (Pithecophaga jefferyi) and hawk-eagles (Spizaetus force leading to the evolution of behavioral traits spp.), reports on the predation of primates by raptors and social systems in primates (van Schaik, 1983; in Asia are limited (Ferguson-Lee and Christie, 2001; Hart, 2007), although there are few case studies on Hart, 2007; Fam and Nijman, 2011). -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

For Peer Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Page 1 of 57 Journal of Morphology provided by Archivio della Ricerca - Università di Pisa 1 2 3 Title: The locomotion of Babakotia radofilai inferred from epiphyseal and diaphyseal 4 5 6 morphology of the humerus and femur. 7 8 9 Damiano Marchi1,2*, Christopher B. Ruff3, Alessio Capobianco, 1,4, Katherine L. Rafferty5, 10 11 Michael B. Habib6, Biren A. Patel2,6 12 13 14 1 15 Department of Biology, University of Pisa, Pisa, Italy, 56126 16 17 2 18 Evolutionary Studies Institute, University of the Witwatersrand, Johannesburg, South 19 20 Africa, WITS 2050 For Peer Review 21 22 23 3 Center for Functional Anatomy and Evolution, Johns Hopkins University School of 24 25 26 Medicine, Baltimore, MD 21111 27 28 4 29 Scuola Normale Superiore, Pisa, Italy, 56126 30 31 5 32 Department of Orthodontics, School of Dentistry, University of Washington, Seattle, WA 33 34 35 98195 36 37 6 38 Department of Cell and Neurobiology, Keck School of Medicine, University of Southern 39 40 California, Los Angeles, CA 90033 41 42 43 Text pages: 28; Bibliography pages: 9; Figures: 6; Tables: 6 Appendices: 1 44 45 46 Running title: Babakotia radofilai postcranial suspensory adaptations 47 48 49 *Corresponding author: 50 51 52 Damiano Marchi 53 54 55 56 Address: Dipartimento di Biologia, Università di Pisa, Via Derna, 1 - ZIP 56126, Pisa - Italy 57 58 59 Ph: +39 050 2211350; Fax: +39 050 2211475 60 1 John Wiley & Sons Journal of Morphology Page 2 of 57 1 2 3 Email: [email protected] -

Habitat Suitability of Proboscis Monkey (Nasalis Larvatus) in Berau Delta, East Kalimantan, Indonesia
BIODIVERSITAS ISSN: 1412-033X Volume 21, Number 11, November 2020 E-ISSN: 2085-4722 Pages: 5155-5163 DOI: 10.13057/biodiv/d211121 Habitat suitability of Proboscis monkey (Nasalis larvatus) in Berau Delta, East Kalimantan, Indonesia TRI ATMOKO1,2,♥, ANI MARDIASTUTI3♥♥, M. BISMARK4, LILIK BUDI PRASETYO3, ♥♥♥, ENTANG ISKANDAR5, ♥♥♥♥ 1Research and Development Institute for Natural Resources Conservation Technology. Jl. Soekarno-Hatta Km 38, Samboja, Samarinda 75271, East Kalimantan, Indonesia. Tel.: +62-542-7217663, Fax.: +62-542-7217665, ♥email: [email protected], [email protected]. 2Program of Primatology, Institut Pertanian Bogor. Jl. Lodaya II No. 5, Bogor 16151, West Java, Indonesia 3Department of Forest Resource Conservation and Ecotourism, Faculty of Forestry and Environment, Institut Pertanian Bogor. Jl. Lingkar Akademik, Kampus IPB Dramaga, Bogor16680, West Java, Indonesia. Tel.: +62-251-8621677, ♥♥email: [email protected], ♥♥♥[email protected] 4Forest Research and Development Center. Jl. Gunung Batu No 5, Bogor 16118, West Java, Indonesia 5Primate Research Center, Institut Pertanian Bogor. Jl. Lodaya II No. 5, Bogor 16151, West Java, Indonesia. Tel./fax.: +62-251-8320417, ♥♥♥♥email: [email protected] Manuscript received: 1 October 2020. Revision accepted: 13 October 2020. Abstract. Atmoko T, Mardiastuti A, Bismark M, Prasetyo LB, Iskandar E. 2020. Habitat suitability of Proboscis Monkey (Nasalis larvatus) in Berau Delta, East Kalimantan, Indonesia. Biodiversitas 21: 5155-5163. Habitat suitability of Proboscis monkey (Nasalis larvatus) in Berau Delta, East Kalimantan, Indonesia. The proboscis monkey (Nasalis larvatus) is an endemic species to Borneo's island and is largely confined to mangrove, riverine, and swamp forests. Most of their habitat is outside the conservation due to degraded and habitat converted. -

Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 000:000–000 (2006) Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S. Thorpe1* and Robin H. Crompton2 1School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK 2Department of Human Anatomy and Cell Biology, University of Liverpool, Liverpool L69 3GE, UK KEY WORDS Pongo pygmaeus; posture; orthograde clamber; forelimb suspend ABSTRACT The Asian apes, more than any other, are and orthograde compressive locomotor modes are ob- restricted to an arboreal habitat. They are consequently an served more frequently. Given the complexity of orangu- important model in the interpretation of the morphological tan positional behavior demonstrated by this study, it is commonalities of the apes, which are locomotor features likely that differences in positional behavior between associated with arboreal living. This paper presents a de- studies reflect differences in the interplay between the tailed analysis of orangutan positional behavior for all age- complex array of variables, which were shown to influence sex categories and during a complete range of behavioral orangutan positional behavior (Thorpe and Crompton [2005] contexts, following standardized positional mode descrip- Am. J. Phys. Anthropol. 127:58–78). With the exception tions proposed by Hunt et al. ([1996] Primates 37:363–387). of pronograde suspensory posture and locomotion, orang- This paper shows that orangutan positional behavior is utan positional behavior is similar to that of the African highly complex, representing a diverse spectrum of posi- apes, and in particular, lowland gorillas. This study sug- tional modes. Overall, all orthograde and pronograde sus- gests that it is orthogrady in general, rather than fore- pensory postures are exhibited less frequently in the pres- limb suspend specifically, that characterizes the posi- ent study than previously reported. -

Controlled Alien Species -Common Name
Controlled Alien Species –Common Name List of Controlled Alien Species Amphibians by Common Name -3 species- (Updated December 2009) Frogs Common Name Family Genus Species Poison Dart Frog, Black-Legged Dendrobatidae Phyllobates bicolor Poison Dart Frog, Golden Dendrobatidae Phyllobates terribilis Poison Dart Frog, Kokoe Dendrobatidae Phyllobates aurotaenia Page 1 of 50 Controlled Alien Species –Common Name List of Controlled Alien Species Birds by Common Name -3 species- (Updated December 2009) Birds Common Name Family Genus Species Cassowary, Dwarf Cassuariidae Casuarius bennetti Cassowary, Northern Cassuariidae Casuarius unappendiculatus Cassowary, Southern Cassuariidae Casuarius casuarius Page 2 of 50 Controlled Alien Species –Common Name List of Controlled Alien Species Mammals by Common Name -437 species- (Updated March 2010) Common Name Family Genus Species Artiodactyla (Even-toed Ungulates) Bovines Buffalo, African Bovidae Syncerus caffer Gaur Bovidae Bos frontalis Girrafe Giraffe Giraffidae Giraffa camelopardalis Hippopotami Hippopotamus Hippopotamidae Hippopotamus amphibious Hippopotamus, Madagascan Pygmy Hippopotamidae Hexaprotodon liberiensis Carnivora Canidae (Dog-like) Coyote, Jackals & Wolves Coyote (not native to BC) Canidae Canis latrans Dingo Canidae Canis lupus Jackal, Black-Backed Canidae Canis mesomelas Jackal, Golden Canidae Canis aureus Jackal Side-Striped Canidae Canis adustus Wolf, Gray (not native to BC) Canidae Canis lupus Wolf, Maned Canidae Chrysocyon rachyurus Wolf, Red Canidae Canis rufus Wolf, Ethiopian -

Ontogeny of Positional Behavior in Captive Silvered Langurs (Trachypithecus Cristatus)
Ontogeny of Positional Behavior in Captive Silvered Langurs (Trachypithecus cristatus) A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for graduation with research distinction in Anthropological Science in the undergraduate colleges of The Ohio State University by Amy Eakins The Ohio State University March 2010 Project Advisor: Professor W. Scott McGraw, Department of Anthropology ABSTRACT Compared to most other mammalian groups, primates are known for the great diversity of positional behavior they exhibit. Their positional repertoire is not static through time, but rather changes with age. As primates age and body size increases, the manner in which animals navigate their environment responds to shifting biomechanical, nutritional, socio-behavioral and reproductive factors. In this study, I examined positional behavior in a colony of captive colobine monkeys, hypothesizing that locomotor and postural diversity will increase with age due to changing physiological and ecological processes. I predicted that as animals mature, their positional diversity will increase as they become more adept at negotiating their three- dimensional environments. I examined age effects on positional behavior in silvered langurs (Trachypithecus cristatus) housed at the Columbus Zoo. Data were collected from January – August 2009 using instantaneous focal animal sampling on a breeding group containing four adults, two juveniles, and one infant. During each scan I recorded the focal animal’s identity, maintenance activity, substrate, and postural (19 categories) or locomotor (12 categories) behavior. Chi-square tests were performed on the data set of 4504 scans. Contrary to expectations, my analyses show that the number of observed positional behaviors did not change significantly with age, although the types of behaviors observed did change.