Healthcare Fraud & Abuse Review 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Group Long Term Disability Insurance
Vendor Evaluation Criteria Response Matrix RFP/RLI/RFQ Number and Title GEN2118079P2 - Group Long Term Disability Insurance Vendor Name Life Insurance Company of North America USAble Life Lincoln Financial Group Metropolitan Life Insurance Company (MLIC) Vendor Address Two Liberty Place 1601 Chestnut Street 4800 Deerwood Campus Parkway, Jacksonville, 100 Liberty Way, Ste. 100, Dover, NH 03820 200 Park AvenueNew York, NY 10166-0188 Philadelphia, PA 19192 FL 32246 Evaluation Criteria Vendor Response LOCATION: (MAXIMUM POINTS 5) Refer to Question 1 GEN2118079P2 Page 1 RFP/RLI/RFQ Number and Title GEN2118079P2 - Group Long Term Disability Insurance Vendor Name Life Insurance Company of North America USAble Life Lincoln Financial Group Metropolitan Life Insurance Company (MLIC) Refer to Vendor’s Business Location Attestation Form and Not applicable. Confirmed. USAble Life has included the completed We have provided the Vendor's Business Location MetLife does not have a principal place of business submit as instructed. "Vendor's Business Location Attestation" form.State of Attestation Form with this proposal. location, also known as the nerve center within Florida Department of Corporations: - Corporate Broward County. A Vendor with a principal place of business location (also known as the nerve center) within Broward County for the Name = USAble Life - Documentation # = last six months, prior to the solicitation submittal, will F01000005275 receive five points; a Vendor not meeting all of the local business requirements will receive zero points. The following applies for a Vendor responding as a Joint Venture (JV): if a member of the JV has 51% or more of the equity and meets all of the local business requirements, the JV will receive three points; if a member of the JV has 30 to 50% of the equity and meets all of the local business requirements, the JV will receive two points; and if a member of the JV has 10% to 29% of the equity and meets all of the local business requirements, the JV will receive one point. -
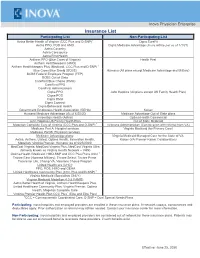
Insurance List
Inova Physician Enterprise Insurance List Participating List Non-Participating List Aetna Better Health of Virginia (CCC Plus and D-SNP)1 Cigna SureFit Aetna PPO, POS and HMO Cigna Medicare Advantage (Inova will be par as of 1/1/21) Aetna Coventry Aetna Coresource Aetna/First Health Anthem PPO (Blue Cross of Virginia) Health First Anthem Healthkeepers (HMO) Anthem Healthkeepers Plus (Medicaid, CCC Plus and D-SNP) 1 Blue Cross Blue Shield (BCBS) Humana (All plans except Medicare Advantage and Military) BCBS Federal Employee Program (FEP) BCBS Out of State CareFirst Blue Choice (HMO) CareFirst PPO CareFirst Administrators Cigna PPO John Hopkins (All plans except US Family Health Plan) Cigna POS Cigna HMO Cigna Connect Cigna Behavioral Health Government Employees Health Association (GEHA) Kaiser Humana Medicare Advantage (As of 6/25/20) Medicare Advantage Out of State plans Innovation Health (Aetna) Optima Health Commercial John Hopkins US Family Health Plan Out of State Medicaid Magellan Complete Care of Virginia (CCC Plus and D-SNP) 1 Veterans Administration (Can be seen with referral from VA) Medicare Part A: Hospital services Virginia Medicaid (for Primary Care) Medicare Part B: Physician services Medicare Advantage plans: Virginia Medicaid Managed Care for the State of VA: Aetna, Anthem, United, Optima Health, Innovation Health, Kaiser (VA Premier-Kaiser Collaboration) Magellan, Virginia Premier, Humana (as of 6/25/2020) MedCost Virginia, MedCost Virginia Plus, MedCost Virginia Ultra (formerly known as Virginia Health Network – VHN) -

CIGNA Corporation (Exact Name of Registrant As Specified in Its Charter)
SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-Q QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended March 31, 2010 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-08323 CIGNA Corporation (Exact name of registrant as specified in its charter) Delaware 06-1059331 (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) Two Liberty Place, 1601 Chestnut Street Philadelphia, Pennsylvania 19192 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code (215) 761-1000 Not Applicable (Former name, former address and former fiscal year, if changed since last report) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes No Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes No Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. -

United States of America Ex Rel. Dr. Peter Rost V. Pfizer
UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS UNITED STATES OF AMERICA ex rel. * DR. PETER ROST, * * Plaintiff, * * v. * Civil Action No. 03-11084-JLT * PFIZER INC and PHARMACIA * CORPORATION, * * Defendants. * MEMORANDUM August 30, 2006 TAURO, J. Plaintiff-Relator, Dr. Peter Rost, (“Plaintiff”) brings this qui tam action against Defendants Pfizer, Inc. and Pharmacia Corporation (collectively “Defendants”), alleging violations of the Federal False Claims Act1 (“FCA”) and similar state statutes.2 Plaintiff asserts that Defendants, through illegal, off-label marketing of the drug Genotropin, knowingly caused the submission of false claims to federal and state health insurance programs. Defendants now move to dismiss 1 See 31 U.S.C. § 3729 et seq. (2006). 2 See California False Claims Act, Cal. Gov’t Code §§ 12650 et seq.; Delaware False Claims and False Reporting Act, Del. Code Ann. tit. 6, §§ 1201 et seq.; District of Columbia Procurement Reform Amendment Act, D.C. Code §§ 2-308-13 et seq.; Florida False Claims Act, Fla. Stat. §§ 68.081 et seq.; Hawaii False Claims Act, Haw. Rev. Stat. §§ 661-21 et seq.; Illinois Whistleblower Reward and Protection Act, 740 Ill. Comp. Stat.175 et seq.; Massachusetts False Claims Law, Mass. Gen. Laws ch. 12, §§ 5 et seq.; Nevada False Claims Act, Nev. Rev. Stat. §§ 357.010 et seq.; Tennessee Medicaid Fraud Prevention Law, Tenn. Code Ann. §§ 71-5-181 et seq.; Texas Medicaid Fraud Prevention Law, Tex. Hum. Res. Code Ann. §§ 36.001 et seq.; Virginia Fraud Against Taxpayers Act, Va. Code Ann. §§ 8.01-216.1 et seq. 1 Plaintiff’s complaint, arguing that this court lacks subject matter jurisdiction and that Plaintiff’s complaint fails to allege fraud with sufficient particularity. -

Commercial Insurance Plan Contracts
At Mayo Clinic's campus in Arizona, Mayo Clinic providers and Mayo Clinic Hospital are contracted with the organizations listed below. Insurance types include: Commercial, International, and Transplant. Your benefit coverage for care provided by Mayo Clinic is determined solely by your insurance company and is based on the provisions of your specific medical benefit plan. Please contact the customer service department on the back of your member identification card to confirm if you have in-network access to Mayo Clinic, as well as your benefit level, for care provided at Mayo Clinic. You may also contact Mayo Clinic directly at 844-217-9591 (Toll-Free) or 507-266-0909 (International) for more details about the information listed below. Commercial Insurance Plan Contracts Aetna • Aetna Choice POS II • Aetna Choice • Aetna Choice Plus • Aetna Choice POS • Aetna Choice POS (Aetna HealthFund) • Aetna Choice POS (Open Access) • Aetna Choice POS II (Aetna HealthFund) • Aetna Choice POS II (Open Access) • Aetna Health Network Only (Open Access) • Aetna Health Network Option (Open Access) • Aetna HealthFund Aetna Health Network Only (Open Access) • Aetna HealthFund Aetna Health Network Option (Open Access) • Aetna Open Access • Aetna Open Access Elect Choice EPO (Aetna HealthFund) • Aetna Open Access Managed Choice POS (Aetna HealthFund) • Aetna PPO • Aetna Select • Aetna Select Open Access • Aetna Select Open Access/Aetna Select • Aetna Signature Administrators (ASA) • Aetna Student Health Plans • Aexcel Aetna Select • Aexcel Aetna Select (Open -

Cigna Health and Life Insurance Co.: Open Access Plus in Coverage For: Individual/Individual + Family | Plan Type: OAP
Summary of Benefits and Coverage: What this Plan Covers & What You Pay For Covered Services Coverage Period: 07/01/2019 - 06/30/2020 Cigna Health and Life Insurance Co.: Open Access Plus IN Coverage for: Individual/Individual + Family | Plan Type: OAP The Summary of Benefits and Coverage (SBC) document will help you choose a health plan. The SBC shows you how you and the plan would share the cost for covered health care services. NOTE: Information about the cost of this plan (called the premium) will be provided separately. This is only a summary. For more information about your coverage, or to get a copy of the complete terms of coverage, go online at www.cigna.com/sp. For general definitions of common terms, such as allowed amount, balance billing, coinsurance, copayment, deductible, provider, or other underlined terms see the Glossary. You can view the Glossary at https://www.healthcare.gov/sbc-glossary or call 1-800-Cigna24 to request a copy. Important Questions Answers Why This Matters: What is the overall See the Common Medical Events chart below for your costs for $0 deductible? services this plan covers. Are there services covered You will have to meet the deductible before the plan pays for any before you meet your No. services. deductible? Are there other deductibles No. You don't have to meet deductibles for specific services. for specific services? The out-of-pocket limit is the most you could pay in a year for What is the out-of-pocket covered services. If you have other family members in this plan, For in-network providers $2,000/individual or $4,000/family limit for this plan? they have to meet their own out-of-pocket limits until the overall family out-of-pocket limit has been met. -

Largest 25 Whistleblower Rewards Paid Under the False Claims Act
Top 25 Largest Whistleblower Rewards paid under the False Claims Act and Qui Tam Whistleblower Reward Program Here is a list of the top 25 largest Federal and State False Claims Act qui tam whistleblower reward cases. The government pays rewards to whistleblowers a portion ranging from 15% to 25% of the civil False Claims Act recovery. More details about whistleblower rewards can be found at www.HowToReportFraud.com. Rank Company FCA Settlement Reward Date 1. GlaxoSmithKlinei $2 billion $300 million≈ July 2012 2. Bank of Americaii $1.85 billion $275 million≈ November 2014 3. Johnson & Johnsoniii $1.72 billion $167 million November 2013 4. Pfizeriv $1 billion $102 million August 2009 5. Tenet Heathcarev $900 million $150 million June 2006 6. Abbott Labsvi $800 million $84 million May 2012 7. Eli Lillyvii $800 million $79 million January 2009 8. HCAviii $745 million $150 million December 2000 9. Merckix $650 million $100 million≈ February 2008 10. HCAx $641 million $151 million June 2003 11. JP Morganxi $614 million $90 million≈ February 2014 12. Amgenxii $612 million $90 million≈ December 2012 13. GlaxoSmithKilnexiii $600 million $90 million≈ October 2010 14. Seronoxiv $567 million $50 million October 2005 15. TAPxv $540 million $95 million October 2001 16. New Yorkxvi $540 million $10 million* July 2009 17. AstraZenecaxvii $520 million $45 million April 2010 18. Abbott Labsxviii $414 million $60 million≈ July 2003 19. Freseniusxix $385 million $65 million January 2000 20. NMExx $379 million $65 million July 1994 21. Cephalonxxi $375 million $46 million September 2008 22. Ranbaxyxxii $350 million $49 million May 2013 23. -

Health Care Enforcement 2020 Year in Review & 2021 Outlook
HEALTH CARE ENFORCEMENT 2020 Year in Review & 2021 Outlook TABLE OF CONTENTS Introduction ......................................................................................................................................3 Statistical Trends in Civil False Claims Act Litigation in 2020 .................................................................4 Qui Tam Case Volume Decreased While Government-Initiated Matters Increased .................................4 Hospitals and Physicians Continue to Be the Leading Targets of Qui Tam Cases ...................................7 Current and Former Employees Continue to Bring the Vast Majority of Health Care Qui Tam Cases ...............................................................................................................................7 Health Care Qui Tam Suits Are Concentrated in Major Metropolitan Areas ............................................8 Health Care Fraud Enforcement Priorities in 2020 ................................................................................9 Opioids .........................................................................................................................................9 Purdue Pharma ............................................................................................................................9 Indivior Solutions ....................................................................................................................... 10 Practice Fusion ......................................................................................................................... -

Qui Tam Relators, the First Amendment, and the False Claims Act Joshua Patrick Mahoney [email protected]
University of Chicago Legal Forum Volume 2012 | Issue 1 Article 13 Qui Tam Relators, the First Amendment, and the False Claims Act Joshua Patrick Mahoney [email protected] Follow this and additional works at: http://chicagounbound.uchicago.edu/uclf Recommended Citation Mahoney, Joshua Patrick () "Qui Tam Relators, the First Amendment, and the False Claims Act," University of Chicago Legal Forum: Vol. 2012: Iss. 1, Article 13. Available at: http://chicagounbound.uchicago.edu/uclf/vol2012/iss1/13 This Comment is brought to you for free and open access by Chicago Unbound. It has been accepted for inclusion in University of Chicago Legal Forum by an authorized administrator of Chicago Unbound. For more information, please contact [email protected]. Comments Qui Tam Relators, the First Amendment, and the False Claims Act Joshua Patrick Mahoneyt INTRODUCTION The False Claims Act (FCA)1 allows a private citizen to file suit "for the person and for the United States government." 2 The citizen sues to recover damages for "false or fraudulent claim[s]" submitted to the government for payment.3 While the Attorney General is also authorized to bring claims under the FCA, 4 pri- vate citizen suits, also called "qui tam"5 suits, comprise a signifi- cant majority of FCA claims filed.6 Qui tam suits account for the largest portion of funds recovered under the FCA.7 To bring a qui t BA 2009, University of Northern Iowa; JD Candidate 2013, The University of Chicago Law School. 1 31 USC §§ 3729-33. 2 31 USC § 3730(b)(1). 3 31 USC § 3729(a). -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

False Claims Act Practice Guide 2021 ©2021 Smith Pachter Mcwhorter PLC
False Claims Act Practice Guide 2021 ©2021 Smith Pachter McWhorter PLC. This publication is not intended to provide legal advice but to provide information on legal matters. Transmission is not intended to create and receipt does not establish an attorney-client relationship. Readers should seek specific legal advice before taking any action with respect to matters mentioned in this publication. SMITH PACHTER McWHORTER PLC Table of Contents Introduction Part One: FCA Statutory Framework and Legal Elements A. FCA Statute and Elements of Proof .............................................................................................. 1 B. Elements of Proof and Judicial Interpretations of Terms ................................................................... 2 C. Qui Tam Provision ................................................................................................................... 4 D. FCA’s Statute of Limitations ....................................................................................................... 6 E. Damages ............................................................................................................................... 6 Part Two: Risk Areas and Enforcement A. Theories of Liability ................................................................................................................. 8 1. Improper Performance on Deliverables ................................................................................... 9 2. Truth in Negotiations Act Violations ...................................................................................... -

Astrazeneca to Pay $520 Million for Off-Label Drug Marketing
U.S. Department of Justice United States Attorney Eastern District of Pennsylvania 615 Chestnut Street Suite 1250 Philadelphia, Pennsylvania 19106-4476 (215) 861-8200 April 27, 2010 PHARMACEUTICAL COMPANY ASTRAZENECA TO PAY $520 MILLION FOR OFF-LABEL DRUG MARKETING WASHINGTON – The Department of Justice today announced a $520 million civil settlement with pharmaceutical company AstraZeneca LP and AstraZeneca Pharmaceuticals, LP (AstraZeneca) to resolve allegations made under the civil False Claims Act that AstraZeneca illegally marketed the anti-psychotic drug Seroquel for uses not approved as safe and effective by the Food and Drug Administration (FDA). Such unapproved uses are also known as “off-label” uses because they are not included in the drug’s FDA approved product label. United States Attorney General Eric Holder, Assistant Attorney General for the Civil Division Tony West, and United States Attorney Michael L. Levy of the Eastern District of Pennsylvania announced the settlement today. They were joined by Special Agent-in-Charge Nick DiGiulio of the Office of Inspector General of the Department of Health and Human Services. AstraZeneca, headquartered in Wilmington, Delaware, signed a civil settlement to resolve allegations that by marketing Seroquel for unapproved uses, the company caused false claims for payment to be submitted to federal insurance programs including Medicaid, Medicare, and TRICARE programs, and to the Department of Veterans Affairs, the Federal Employee Health Benefits Program, and the Bureau of Prisons. The civil settlement agreement provides that AstraZeneca will pay up to $520 million to the federal government and the states to resolve civil allegations originally brought in a lawsuit under the qui tam provisions of the federal False Claims Act and various state False Claims Act statutes.