Impacts of Climate Change and Ocean Acidification on Indian Ocean Tunas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
North America Other Continents
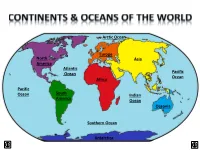
Arctic Ocean Europe North Asia America Atlantic Ocean Pacific Ocean Africa Pacific Ocean South Indian America Ocean Oceania Southern Ocean Antarctica LAND & WATER • The surface of the Earth is covered by approximately 71% water and 29% land. • It contains 7 continents and 5 oceans. Land Water EARTH’S HEMISPHERES • The planet Earth can be divided into four different sections or hemispheres. The Equator is an imaginary horizontal line (latitude) that divides the earth into the Northern and Southern hemispheres, while the Prime Meridian is the imaginary vertical line (longitude) that divides the earth into the Eastern and Western hemispheres. • North America, Earth’s 3rd largest continent, includes 23 countries. It contains Bermuda, Canada, Mexico, the United States of America, all Caribbean and Central America countries, as well as Greenland, which is the world’s largest island. North West East LOCATION South • The continent of North America is located in both the Northern and Western hemispheres. It is surrounded by the Arctic Ocean in the north, by the Atlantic Ocean in the east, and by the Pacific Ocean in the west. • It measures 24,256,000 sq. km and takes up a little more than 16% of the land on Earth. North America 16% Other Continents 84% • North America has an approximate population of almost 529 million people, which is about 8% of the World’s total population. 92% 8% North America Other Continents • The Atlantic Ocean is the second largest of Earth’s Oceans. It covers about 15% of the Earth’s total surface area and approximately 21% of its water surface area. -

A COMPARATIVE ACCOUNT of the SMALL PELAGIC FISHERIES in the APFIC REGION by M
A COMPARATIVE ACCOUNT OF THE SMALL PELAGIC FISHERIES IN THE APFIC REGION by M. Devaraj and E. Vivekanandan Central Marine Fisheries Research Institute Cocbin-682014, India Abstract The production of the small pe/agics in the APFIC region was 1.2 mt/sq. km during 1995. Among the four areas in the region, the small pe/agics have registered (i) the maximum annual fluctuations in the western Indian Ocean; (ii) the highest increase duri'}i the past two decades along the west coast of Thailand in the eastern Indian Ocean; and (iii) the consistent decline in the landings during the past one decade along the Japanese coast in the northwest Pacific Ocean. The short rnackerels emerged as the largest fishery in the APFlC region, fom'ing 19.5% of the landings of the small pelagics in 1995. The group consisting afthe sardines and the anchovies has shown clear signs of decline during the past one decade in almost the entire region. Most of the small pelagics have unique biological characteristics such as fast growth, short longevity, late maturity, high nalllral mortality, shoaling behaviour, high fecundity and severe recruitment fluctuations. As many species of the small pelagics undertake migration, collaborative research programmes and close coordination are required among the APFle countries for the stock assessment of all the major species. The management measures under implementation in these countries have been reviewed, with suggestions for regional cooperation for the management of the stocks of the small pelagics. INTRODUCTION The Asia-Pacific Fishery Commission covers four oceanic areas, which have been classified by the FAO as the western Indian Ocean (FAO Statistical Area 51), eastern Indian Ocean (Area 57) , northwest Pacific Ocean (Area 61) and western central Pacific Ocean (Area 71). -

Effects of Europe's Commercial Expansion Into the Indian Ocean On
Effects of Europe’s Commercial Expansion into the Indian Ocean on Asian and African Coastal Economies, 1600-1650 Johannes Lang 8GRG Neulandschule Grinzing Alfred Wegener-Gasse 10-12 1190 Wien 29.1.2016 Betreut von Mag. Ruth Schabauer Abstract This work examines the impact of Europeans’ commercial expansion into the Indian Ocean on the local Asian and African economies between 1600 and 1650. By studying this historically important period of time, we can also gain a deeper understanding of modern globalization and of Europe’s continuing political and economic influence today. The different consequences for the various regions bordering the Indian Ocean are compared, contrasted, and evaluated. For my research I use primarily books and articles but also rely on the analysis of economic data. Epic poems from Mughal writers as well as modern studies are included so that the reader may gain thorough insights into the topic. As I try to tell history from an Afro-Asian perspective, I let both 17th century and contemporary voices native to the Indian Ocean have their say. I conclude in my study that the consequences of trade with the Europeans differed greatly between the heterogeneous regions. The nature of these consequences depended on the socioeconomic structure as well as on the environmental particularities of the regions in question. Some economies profited from the new situation; others suffered from the altered trade system. Interestingly, many effects of 17th century globalization, such as increased competition with countries far away and a heightened reliance on foreign trade, are visible also in today’s process of globalization. -

Longtail Tuna (Thunnus Tonggol)
I & I NSW WILD FISHERIES RESEARCH PROGRAM Longtail Tuna (Thunnus tonggol) EXPLOITATION STATUS UNDEFINED A coastal tuna species for which the recreational fishery is probably more significant than the commercial fishery. There are few useful data with which to establish status. SCIENTIFIC NAME STANDARD NAME COMMENT Previously, but incorrectly called northern Thunnus tonggol longtail tuna bluefin tuna. Thunnus tonggol Image © Bernard Yau Background The longtail tuna reaches maturity at lengths of around 60-70 cm, and spawning takes place The longtail tuna (Thunnus tonggol) inhabits during the summer months. The main diet of continental shelf and ocean waters in warm the longtail tuna consists of small pelagic and temperate and tropical regions of the Indo-west demersal fish, but also includes crustaceans and Pacific. It is a common species in Queensland cephalopods. waters but during the summer it can be found as far south as Twofold Bay in southern NSW. Because of their rapid acceleration, longtail Previously called ‘northern bluefin tuna’ in tuna are highly regarded as sports fish but Australia, the longtail tuna is a relatively small, their very dark flesh gives them a low market slender species that grows to a weight of acceptance. Since about 2000 the NSW 36 kg and length of 136 cm; it is more commercial catch has been less than 2 t per commonly 80-90 cm and 10-15 kg. In year, with most taken by the Ocean Trap and comparison, the true ‘northern bluefin’ (Thunnus Line Fishery and very small amounts reported orientalis) can exceed 500 kg in weight and by the Ocean Hauling and Estuary General reach almost 300 cm in length. -

India in the Indian Ocean Donald L
Naval War College Review Volume 59 Article 6 Number 2 Spring 2006 India in the Indian Ocean Donald L. Berlin Follow this and additional works at: https://digital-commons.usnwc.edu/nwc-review Recommended Citation Berlin, Donald L. (2006) "India in the Indian Ocean," Naval War College Review: Vol. 59 : No. 2 , Article 6. Available at: https://digital-commons.usnwc.edu/nwc-review/vol59/iss2/6 This Article is brought to you for free and open access by the Journals at U.S. Naval War College Digital Commons. It has been accepted for inclusion in Naval War College Review by an authorized editor of U.S. Naval War College Digital Commons. For more information, please contact [email protected]. Color profile: Generic CMYK printer profile Composite Default screen Berlin: India in the Indian Ocean INDIA IN THE INDIAN OCEAN Donald L. Berlin ne of the key milestones in world history has been the rise to prominence Oof new and influential states in world affairs. The recent trajectories of China and India suggest strongly that these states will play a more powerful role in the world in the coming decades.1 One recent analysis, for example, judges that “the likely emergence of China and India ...asnewglobal players—similar to the advent of a united Germany in the 19th century and a powerful United States in the early 20th century—will transform the geopolitical landscape, with impacts potentially as dramatic as those in the two previous centuries.”2 India’s rise, of course, has been heralded before—perhaps prematurely. How- ever, its ascent now seems assured in light of changes in India’s economic and political mind-set, especially the advent of better economic policies and a diplo- macy emphasizing realism. -

ICRI Indian Ocean Factsheet
Indian Ocean Factsheet Communicating the Economic and Social Importance of Coral Reefs for Indian Ocean countries This fact sheet will provide you with information extracted from economic studies Indian Ocean Factsheet BASICS Coral reefs are among the most productive ecosystems on the planet. They cover less than 1% of the ocean floor but support 25% of ocean life. Coral reefs provide beautiful seascapes which allow for a range of recreational activities and improve the attractiveness of the country for international tourism markets. Coral reefs absorb a huge amount of swell energy from waves, protecting lives, coastal properties and beaches from flooding events and hurricanes. Many fisheries exist only due to the presence of coral reefs, whether as nurseries or adult habitats. This sector provides food and incomes for fishers and associated industries. More than 65% of reefs in the region are at risk from local threats, with one-third rated at high or very high risk. Primary threats are man made - unsustainable fishing, land based sources of marine pollution (including sedimentation) and more recently Global Climate Change. Local solutions exist !! Developing networks of Marine Protected Areas, implementing sustainable fishing practices (especially protecting herbivores) and improving water quality are local actions that could increase the resilience of coral reefs to global threats. Reefs at Risk in the Indian Ocean Indian Ocean Factsheet REGIONAL DATA Estimates show the 28,000 km2 of reefs in the Indian Ocean provide tangible benefits of at least US$2b annually to the economies of the countries. Tourism benefits represent 70% of this value with Fisheries representing the remainer. -

Why Study Bycatch? an Introduction to the Theme Section on Fisheries Bycatch
Vol. 5: 91–102, 2008 ENDANGERED SPECIES RESEARCH Printed December 2008 doi: 10.3354/esr00175 Endang Species Res Published online December xx, 2008 Contribution to the Theme Section ‘Fisheries bycatch problems and solutions’ OPENPEN ACCESSCCESS Why study bycatch? An introduction to the Theme Section on fisheries bycatch Candan U. Soykan1,*, Jeffrey E. Moore2, Ramunas ¯ 5ydelis2, Larry B. Crowder2, Carl Safina3, Rebecca L. Lewison1 1Biology Department, San Diego State University, 5500 Campanile Dr., San Diego, California 92182-4614, USA 2Center for Marine Conservation, Nicholas School of the Environment, Duke University Marine Laboratory, 135 Duke Marine Lab Road, Beaufort,North Carolina 28516, USA 3Blue Ocean Institute, PO Box 250, East Norwich, New York 11732, USA ABSTRACT: Several high-profile examples of fisheries bycatch involving marine megafauna (e.g. dolphins in tuna purse-seines, albatrosses in pelagic longlines, sea turtles in shrimp trawls) have drawn attention to the unintentional capture of non-target species during fishing operations, and have resulted in a dramatic increase in bycatch research over the past 2 decades. Although a number of successful mitigation measures have been developed, the scope of the bycatch problem far exceeds our current capacity to deal with it. Specifically, we lack a comprehensive understanding of bycatch rates across species, fisheries, and ocean basins, and, with few exceptions, we lack data on demographic responses to bycatch or the in situ effectiveness of existing mitigation measures. As an introduction to this theme section of Endangered Species Research ‘Fisheries bycatch: problems and solutions’, we focus on 5 bycatch-related questions that require research attention, building on exam- ples from the current literature and the contributions to this Theme Section. -

Ecosystem-Based Fisheries Management Improving the Resilience of Ocean Ecosystems to Support Fish Populations, Coastal Communities
A brief from March 2014 Ecosystem-Based Fisheries Management Improving the resilience of ocean ecosystems to support fish populations, coastal communities The fish on the end of your line, the little forage fish that feed the big fish, the corals that build reef habitats, and the catch of the day in your favorite restaurant are interconnected parts of a vibrant ocean ecosystem. Ensuring the long-term health of important marine species will depend upon our ability to understand and account for the interactions among those species, their environment, and the people who rely upon them for food, commerce, and sport. This comprehensive approach, called ecosystem-based fisheries management, is needed to conserve the healthy ecosystems essential to the sustainability of our fisheries and to deal with the increasingly complex challenges facing our oceans. The United States is a global leader in fisheries management and has made great strides in ending overfishing (the problem of catching fish faster than they can reproduce) and rebuilding vulnerable populations under the Magnuson-Stevens Fishery Conservation and Management Act—the primary law governing U.S. ocean fisheries. In many regions of our nation, this progress has helped reestablish more abundant fish populations and created economic benefits for the fishing industry and coastal communities. Core conservation policies added to the law in 1996 and 2007 are fundamental to improving individual fish populations and returning value to fishermen. We must maintain them as the foundation for sustainable management. But the United States should make the transition from a species-by-species approach to ecosystem-based fisheries management to meet the rising and urgent challenges of damaged ocean environments and dynamic, changing oceans. -

A Global Valuation of Tuna an Update February 2020 (Final)
Netting Billions: a global valuation of tuna an update February 2020 (Final) ii Report Information This report has been prepared with the financial support of The Pew Charitable Trusts. The views expressed in this study are purely those of the authors. The content of this report may not be reproduced, or even part thereof, without explicit reference to the source. Citation: Macfadyen, G., Huntington, T., Defaux, V., Llewellin, P., and James, P., 2019. Netting Billions: a global valuation of tuna (an update). Report produced by Poseidon Aquatic Resources Management Ltd. Client: The Pew Charitable Trusts Version: Final Report ref: 1456-REG/R/02/A Date issued: 7 February 2020 Acknowledgements: Our thanks to the following consultants who assisted with data collection for this study: Richard Banks, Sachiko Tsuji, Charles Greenwald, Heiko Seilert, Gilles Hosch, Alicia Sanmamed, Anna Madriles, Gwendal le Fol, Tomasz Kulikowski, and Benoit Caillart. 7 February 2020 iii CONTENTS 1. BACKGROUND AND INTRODUCTION ................................................................... 1 2. STUDY METHODOLOGY ......................................................................................... 3 3. TUNA LANDINGS ..................................................................................................... 5 3.1 METHODOLOGICAL ISSUES ....................................................................................... 5 3.2 RESULTS ............................................................................................................... -

The Complicating Sea: the Indian Ocean As Method
The Complicating Sea: The Indian Ocean as Method Isabel Hofmeyr Comparative Studies of South Asia, Africa and the Middle East, Volume 32, Number 3, 2012, pp. 584-590 (Article) Published by Duke University Press For additional information about this article http://muse.jhu.edu/journals/cst/summary/v032/32.3.hofmeyr.html Access provided by University Of Pennsylvania (13 May 2014 11:12 GMT) The Complicating Sea: The Indian Ocean as Method Isabel Hofmeyr cross a number of domains, the Indian Ocean has moved to the fore. For interna- tional relations experts and foreign policy commentators, the Indian Ocean world represents a strategic arena where the forces shaping a post- American world intersect most visibly. These include the rise of India and China as major economic powers and Sino- Indian competition over oil sea- lanes and African markets and minerals. In a recent book Monsoon: The Indian Ocean and the Future of American Power, security analyst Robert Kaplan describes the Indian Ocean as a zone “where global power dynamics will be revealed.” 1 It is the “coming strategic arena of the twenty- first century.” 2 Within the academy, as transnational and oceanic forms of analysis become more prom- inent, the Indian Ocean attracts attention, especially as a domain that offers rich possibilities for working beyond the templates of the nation- state and area studies. Importantly, the Indian Ocean makes visible a range of lateral networks that fall within the Third World or Global South. It is hence of particular relevance to those pursuing post – area studies scholarship and has much to say to the themes of this special issue on comparative literature across Africa, the Middle East, and South Asia. -

Coastal Upwelling Revisited: Ekman, Bakun, and Improved 10.1029/2018JC014187 Upwelling Indices for the U.S
Journal of Geophysical Research: Oceans RESEARCH ARTICLE Coastal Upwelling Revisited: Ekman, Bakun, and Improved 10.1029/2018JC014187 Upwelling Indices for the U.S. West Coast Key Points: Michael G. Jacox1,2 , Christopher A. Edwards3 , Elliott L. Hazen1 , and Steven J. Bograd1 • New upwelling indices are presented – for the U.S. West Coast (31 47°N) to 1NOAA Southwest Fisheries Science Center, Monterey, CA, USA, 2NOAA Earth System Research Laboratory, Boulder, CO, address shortcomings in historical 3 indices USA, University of California, Santa Cruz, CA, USA • The Coastal Upwelling Transport Index (CUTI) estimates vertical volume transport (i.e., Abstract Coastal upwelling is responsible for thriving marine ecosystems and fisheries that are upwelling/downwelling) disproportionately productive relative to their surface area, particularly in the world’s major eastern • The Biologically Effective Upwelling ’ Transport Index (BEUTI) estimates boundary upwelling systems. Along oceanic eastern boundaries, equatorward wind stress and the Earth s vertical nitrate flux rotation combine to drive a near-surface layer of water offshore, a process called Ekman transport. Similarly, positive wind stress curl drives divergence in the surface Ekman layer and consequently upwelling from Supporting Information: below, a process known as Ekman suction. In both cases, displaced water is replaced by upwelling of relatively • Supporting Information S1 nutrient-rich water from below, which stimulates the growth of microscopic phytoplankton that form the base of the marine food web. Ekman theory is foundational and underlies the calculation of upwelling indices Correspondence to: such as the “Bakun Index” that are ubiquitous in eastern boundary upwelling system studies. While generally M. G. Jacox, fi [email protected] valuable rst-order descriptions, these indices and their underlying theory provide an incomplete picture of coastal upwelling. -

Other Processes Regulating Ecosystem Productivity and Fish Production in the Western Indian Ocean Andrew Bakun, Claude Ray, and Salvador Lluch-Cota
CoaStalUpwellinO' and Other Processes Regulating Ecosystem Productivity and Fish Production in the Western Indian Ocean Andrew Bakun, Claude Ray, and Salvador Lluch-Cota Abstract /1 Theseasonal intensity of wind-induced coastal upwelling in the western Indian Ocean is investigated. The upwelling off Northeast Somalia stands out as the dominant upwelling feature in the region, producing by far the strongest seasonal upwelling pulse that exists as a; regular feature in any ocean on our planet. It is surmised that the productive pelagic fish habitat off Southwest India may owe its particularly favorable attributes to coastal trapped wave propagation originating in a region of very strong wind-driven offshore trans port near the southern extremity of the Indian Subcontinent. Effects of relatively mild austral summer upwelling that occurs in certain coastal ecosystems of the southern hemi sphere may be suppressed by the effects of intense onshore transport impacting these areas during the opposite (SW Monsoon) period. An explanation for the extreme paucity of fish landings, as well as for the unusually high production of oceanic (tuna) fisheries relative to coastal fisheries, is sought in the extremely dissipative nature of the physical systems of the region. In this respect, it appears that the Gulf of Aden and some areas within the Mozambique Channel could act as important retention areas and sources of i "see6stock" for maintenance of the function and dillersitv of the lamer reoional biolooical , !I ecosystems. 103 104 large Marine EcosySlIlms ofthe Indian Ocean - . Introduction The western Indian Ocean is the site ofsome of the most dynamically varying-. large marine ecosystems (LMEs) that exist on our planet.