Medical Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Focal Spot, Spring 2006
Washington University School of Medicine Digital Commons@Becker Focal Spot Archives Focal Spot Spring 2006 Focal Spot, Spring 2006 Follow this and additional works at: http://digitalcommons.wustl.edu/focal_spot_archives Recommended Citation Focal Spot, Spring 2006, April 2006. Bernard Becker Medical Library Archives. Washington University School of Medicine. This Book is brought to you for free and open access by the Focal Spot at Digital Commons@Becker. It has been accepted for inclusion in Focal Spot Archives by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. SPRING 2006 VOLUME 37, NUMBER 1 *eiN* i*^ MALLINCKRC RADIOLO AJIVERSITY *\ irtual Colonoscopy: a Lifesaving Technology ^.IIMi.|j|IUII'jd-H..l.i.|i|.llJ.lii|.|.M.; 3 2201 20C n « ■ m "■ ■ r. -1 -1 NTENTS FOCAL SPOT SPRING 2006 VOLUME 37, NUMBER 1 MIR: 75 YEARS OF RADIOLOGY EXPERIENCE In the early 1900s, radiology was considered by most medical practitioners as nothing more than photography. In this 75th year of Mallinckrodt Institute's existence, the first of a three-part series of articles will chronicle the rapid advancement of radiol- ogy at Washington University and the emergence of MIR as a world leader in the field of radiology. THE METABOLISM OF THE DIABETIC HEART More diabetic patients die from cardiovascular disease than from any other cause. Researchers in the Institute's Cardiovascular Imaging Laboratory are finding that the heart's metabolism may be one of the primary mechanisms by which diseases such as diabetes have a detrimental effect on heart function. VIRTUAL C0L0N0SC0PY: A LIFESAVING TECHNOLOGY More than 55,000 Americans die each year from cancers of the colon and rectum. -

Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, Phd, Jimmy H
Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, PhD, Jimmy H. Hara, MD, Irving M. Rasgon, MD, and Virginia A. Clark, PhD Los Angeles, California Background. Although the American Cancer Society and tients with lesions were referred for colonoscopy; addi others haw established guidelines for colorectal cancer tional lesions were found in 14%. A total of 62 lesions screening, questions of who and how to screen still exist. were discovered, including tubular adenomas, villous Methods. A 60-crn flexible sigmoidoscopy was per adenomas, tubular villous adenomas (23 of the adeno formed on 1000 asymptomatic patients, 45 years of mas with atypia), and one adenocarcinoma. The high age or older, with negative fecal occult blood tests, est percentage of lesions discovered were in the sig who presented for routine physical examinations. Pa moid colon and the second highest percentage were in tients with clinically significant lesions were referred for the ascending colon. colonoscopy. The proportion of lesions that would not Conclusions. The 60-cm flexible sigmoidoscope was have been found if the 24-cm rigid or the 30-cm flexi able to detect more lesions than either the 24-cm or ble sigmoidoscope had been used was identified. 30-cm sigmoidoscope when used in asymptomatic pa Results. Using the 60-cm flexible sigmoidoscope, le tients, 45 years of age and over, with negative fecal oc sions were found in 3.6% of the patients. Eighty per cult blood tests. When significant lesions are discovered cent of the significant lesions were beyond the reach of by sigmoidoscopy, colonoscopy should be performed. -
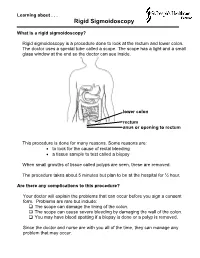
What Is a Rigid Sigmoidoscopy?
Learning about . Rigid Sigmoidoscopy What is a rigid sigmoidoscopy? Rigid sigmoidoscopy is a procedure done to look at the rectum and lower colon. The doctor uses a special tube called a scope. The scope has a light and a small glass window at the end so the doctor can see inside. lower colon rectum anus or opening to rectum This procedure is done for many reasons. Some reasons are: • to look for the cause of rectal bleeding • a tissue sample to test called a biopsy When small growths of tissue called polyps are seen, these are removed. The procedure takes about 5 minutes but plan to be at the hospital for ½ hour. Are there any complications to this procedure? Your doctor will explain the problems that can occur before you sign a consent form. Problems are rare but include: The scope can damage the lining of the colon. The scope can cause severe bleeding by damaging the wall of the colon. You may have blood spotting if a biopsy is done or a polyp is removed. Since the doctor and nurse are with you all of the time, they can manage any problem that may occur. What do I need to do to get ready at home? 4 to 5 days before your procedure: Taking medications: Your doctor may want you to stop taking certain medications 4 to 5 days before the procedure. If you need to stop any medications, your doctor will tell you during the office visit. If you have any questions, call the doctor’s office. Buying a Fleet enema: Your bowel must be clean and empty of waste material before this procedure. -

Rectal Water Contrast Transvaginal Ultrasound Versus Double-Contrast Barium Enema in the Diagnosis of Bowel Endometriosis
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2017-017216 on 7 September 2017. Downloaded from Rectal water contrast transvaginal ultrasound versus double-contrast barium enema in the diagnosis of bowel endometriosis Jipeng Jiang, Ying Liu, Kun Wang, Xixiang Wu, Ying Tang To cite: Jiang J, Liu Y, Wang K, ABSTRACT Strengths and limitations of this study et al. Rectal water contrast Objectives The aim of study was to compare the transvaginal ultrasound versus accuracy between rectal water contrast transvaginal ► This is the first comparison of the accuracy between double-contrast barium enema ultrasound (RWC-TVS) and double-contrast barium enema in the diagnosis of bowel rectal water contrast transvaginal ultrasound (RWC- (DCBE) in evaluating the bowel endometriosis presence as endometriosis. BMJ Open TVS) and double-contrast barium enema (DCBE) in well as its extent. 2017;7:e017216. doi:10.1136/ the diagnosis of bowel endometriosis. Design and setting 198 patients at reproductive age with bmjopen-2017-017216 ► This study demonstrated RWC-TVS as a very reliable suspicious bowel endometriosis were included. Physicians technique to determine the bowel endometriosis ► Prepublication history for in two groups specialised at endometriosis performed presence and extent and it has similar accuracy to this paper is available online. RWC-TVS as well as DCBE before laparoscopy and both To view these files please visit that of DCBE. groups were blinded to other groups’ results. Findings the journal online (http:// dx. doi. ► We demonstrate that DCBE is related to more from RWC-TVS or DCBE were compared with histological org/ 10. 1136/ bmjopen- 2017- tolerance than RWC-TVS. -

04. EDITORIAL 1/2/06 10:34 Página 853
04. EDITORIAL 1/2/06 10:34 Página 853 1130-0108/2005/97/12/853-859 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS REV ESP ENFERM DIG (Madrid) Copyright © 2005 ARÁN EDICIONES, S. L. Vol. 97, N.° 12, pp. 853-859, 2005 Cost-effectiveness of abdominal ultrasonography in the diagnosis of colorectal carcinoma Colorectal cancer (CRC) is a most common neoplasm, and the second leading cause of cancer-related death. CRC was responsible for 11% of cancer-related deaths in males, and for 15% of cancer-related deaths in females according to data for year 2000. Most recent data reported in Spain on death causes in 2002 suggest that CRC was responsible for 12,183 deaths (6,896 males with a mean age of 70 years, and 5,287 women with a mean age of 71 years). In these tumors, mortality data do not reflect the true incidence of this disease, since survival has improved in recent years, particularly in younger individuals. In contrast to other European countries, Spain ranks in an intermediate position in terms of CRC-re- lated incidence and mortality. This risk clearly increases with age, with a notori- ous rise in incidence from 50 years of age on. Survival following CRC detection and management greatly depends upon tumor stage at the time of diagnosis; hence the importance of early detection and –because of their malignant poten- tial– of the recognition and excision of colorectal adenomas. Thus, polypectomy and then surveillance are the primary cornerstones in the prevention of CRC (1-4). For primary prevention, fiber-rich diets, physical exercising, and the avoidance of overweight, smoking, and alcohol have been recommended. -

Flexible Sigmoidoscopy with Haemorrhoid Banding
Flexible sigmoidoscopy with haemorrhoid banding You have been referred by your doctor to have a flexible sigmoidoscopy which may also include haemorrhoid banding. If you are unable to keep your appointment, please notify the department as soon as possible. This will allow staff to give your appointment to someone else and they will arrange another date and time for you. This booklet has been written to explain the procedures. This will help you make an informed decision in relation to consenting to the investigation. Please read the booklets and consent form carefully. You will need to complete the enclosed questionnaire. You may be contacted via telephone by a trained endoscopy nurse before the procedure, to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated on your letter. If you have any mobility problems or there is a possibility you could be pregnant please telephone appointments staff on 01284 712748. Please note the appointment time is your arrival time on the unit, and not the time of your procedure. Please remember there will be other patients in the unit who arrive after you, but are taken in for their procedure before you. This is either for medical reasons or they are seeing a different Doctor. Due to the limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/escorts will be contacted once the person is available for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make your stay as comfortable and stress free as possible. -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Flexible Sigmoidoscopy – Outpatients
Flexible sigmoidoscopy – Outpatients You have been referred by your doctor to have a flexible sigmoidoscopy. his information booklet has been written to explain the procedure. This will help you to make an informed decision before consenting to the procedure. If you are unable to attend your appointment please inform us as soon as possible. Please ensure you read this booklet and the enclosed consent form thoroughly. Please also complete the enclosed health questionnaire. You may be contacted by an endoscopy trained nurse before your procedure to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated in your letter. Please note your appointment time is your arrival time on the unit and not the time of your procedure. If you have any mobility issues or if there is a possibility you could be pregnant, please contact the appointment staff on 01284 713551 Please remember there will be other patients in the unit who may arrive after you but are taken in for their procedure before you, this is for medical reasons or they are seeing a different doctor. Due to limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/ escorts will be contacted once you are ready for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make you stay as comfortable and stress free as possible. Source: Endoscopy Reference No: 5035-14 Issue date: 8/1/21 Review date: 8/1/24 Page 1 of 11 Medication If you are taking WARFARIN, CLOPIDOGREL, RIVAROXABAN or any other anticoagulant (blood thinning medication), please contact the appointment staff on 01284 713551, your GP or anticoagulation nurse, as special arrangements may be necessary. -

Virtual Colonoscopy
Virtual Colonoscopy National Digestive Diseases Information Clearinghouse Virtual colonoscopy (VC) uses x rays and • You will be asked to hold your breath computers to produce two- and three- during the scan to avoid distortion on dimensional images of the colon (large the images. intestine) from the lowest part, the rectum, • The scanning procedure is then National all the way to the lower end of the small Institute of repeated with you lying on your Diabetes and intestine and display them on a screen. Digestive stomach. and Kidney The procedure is used to diagnose colon Diseases and bowel disease, including polyps, diver- After the examination, the information ticulosis, and cancer. VC can be performed from the scanner must be processed to NATIONAL INSTITUTES with computed tomography (CT), some- create the computer picture or image of OF HEALTH times called a CAT scan, or with magnetic your colon. A radiologist evaluates the resonance imaging (MRI). results to identify any abnormalities. You may resume normal activity after the VC Procedure procedure, although your doctor may While preparations for VC vary, you will usually be asked to take laxatives or other oral agents at home the day before the pro- cedure to clear stool from your colon. You Conventional Colonoscopy may also be asked to use a suppository to In a conventional colonoscopy, the cleanse your rectum of any remaining fecal doctor inserts a colonoscope—a long, matter. flexible, lighted tube—into the patient’s VC takes place in the radiology department rectum and slowly guides it up through of a hospital or medical center. -

Virtual Colonography
Virtual Colonography Radiologists at the VCU Medical Center were among the first in Virginia to develop, test and adopt for routine use a more patient-friendly way to screen for colon polyps and colon cancer. The noninvasive treatment procedure known medically as - Virtual Colonography (VC) or CT Colonography (CTC) - uses computed tomography (CT) scanning to determine whether or not colon polyps are present. Because Virtual Colonography does not require the use of a colonoscope as in a conventional colonoscopy procedure, it is sometimes referred to as a “Virtual Colonoscopy”. What makes Virtual Colonography an ideal option for screening? Because the procedure is noninvasive, safe, quick (lasting only a few minutes), and does not require sedation, Virtual Colonography is an excellent screening technology, and often preferred by patients over the conventional or standard colonoscopy. There are no risks of complications such as bowel perforation or complications from sedation associated with standard colonoscopy. Patients may return to their usual activities as soon as the test is over. Virtual Colonography also allows for evaluation of the entire colon, something a standard colonoscopy may not be able to do in all cases because normal twists and turns in the large intestines make navigating the colonoscope difficult. Virtual Colonography is accurate in identifying significant polyps and offers an alternative screening option for those who are simply not willing to undergo conventional colonoscopy, which is invasive, can be uncomfortable, and requires sedation. How accurate is Virtual Colonography compared to conventional colonoscopy? Early studies show that Virtual Colonography is comparable to conventional colonoscopy in terms of identifying polyps six millimeters or greater in size. -

Digestive Endoscopy in Five Decades
■ COLLEGE LECTURES Digestive endoscopy in five decades Peter B Cotton ABSTRACT – The world of gastroenterology scopy. So-called semi-flexible gastroscopes were changed forever when flexible endoscopes cumbersome and used infrequently by only a few became available in the 1960s. Diagnostic and enthusiasts. therapeutic techniques proliferated and entered the mainstream of medicine, not without some Diagnostic endoscopy controversy. Success resulted in a huge service demand, with the need to train more endo- The first truly flexible gastroscope was developed in 1 This paper is scopists and to organise large endoscopy units the USA, following pioneering work on fibre-optic 2 based on the Lilly and teams of staff. The British health service light transmission in the UK by Harold Hopkins. Lecture given at struggled with insufficient numbers of consul- However, commercial production of endoscopes was the Royal College tants, other staff and resources, and British rapidly dominated by Japanese companies, building of Physicians on endoscopy fell behind that of most other devel- on their earlier expertise with intragastric cameras. 12 April 2005 by oped countries. This situation is now being My involvement began in 1968, whilst doing bench Peter B Cotton addressed aggressively, with many local and research with Dr Brian Creamer at St Thomas’ MD FRCP FRCS, national initiatives aimed at improving access and Hospital, London. An expert in coeliac disease (and Medical Director, choice, and at promoting and documenting jejunal biopsy), he opined that gastroscopy might Digestive Disease quality. Many more consultants are needed and become useful and legitimate only if it became pos- Center, Medical some should be relieved of their internal medi- sible to take target biopsy specimens – since no one University of South Carolina, cine commitment to focus on their specialist seriously believed what endoscopists said that they Charleston, USA skills. -

Ography C Virtual Colonoscopy for Screening
466 Gut 2004;53:466 Gut: first published as on 11 February 2004. Downloaded from Please visit the Gut website (www.gutjnl.com) for links possible to generate three dimensional ultrasound cholangiograms. to these articles – many to full text. The authors prospectively evaluated the ability of this technique, compared with direct cholangiography (endoscopic retrograde cholangiopancreatography (ERCP)/percutaneous transhepatic cho- langiogram (PTC)) and MRCP, to detect and characterise biliary ....................................................................... obstruction in 40 patients. Experienced operators, who were blinded to the results of the other tests, evaluated images for Pseudo-pseudomembranous collagenous technical adequacy, presence and level of obstruction, and c suspected cause of any stricture. Compared with two dimensional colitis ultrasound, three dimensional analysis improved the assessment of m Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J biliary anatomy in seven of 40 patients. Three dimensional Surg Pathol 2003;27:1375–9. ultrasound however visualised the peripapillary region less well Microscopic colitis has been divided into three types (Warren BF, et (80%) than MRCP (95%) and direct cholangiography (100%) but al. Histopathology 2002;40:374–6), all characterised by watery was superior at demonstrating the gall bladder and biliary tree diarrhoea and minimal mucosal changes at colonoscopy, asso- proximal to a stricture. All techniques were highly sensitive for ciated with an increase in lamina propria lymphocytes and minimal detection of biliary obstruction (100%) and each diagnosed the crypt architectural distortion. Of the three types, lymphocytic colitis likely cause in 90–95% of cases. Three dimensional ultrasound also has an increase in intraepithelial lymphocytes, collagenous detected the correct level of obstruction in 92% of cases compared colitis has a subepithelial collagen band, and microscopic colitis not with 95% for MRCP and 90% for ERCP/PTC.