Jackfish Lake 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Camper's Guide to Alberta Parks
Discover Value Protect Enjoy The Camper’s Guide to Alberta Parks Front Photo: Lesser Slave Lake Provincial Park Back Photo: Aspen Beach Provincial Park Printed 2016 ISBN: 978–1–4601–2459–8 Welcome to the Camper’s Guide to Alberta’s Provincial Campgrounds Explore Alberta Provincial Parks and Recreation Areas Legend In this Guide we have included almost 200 automobile accessible campgrounds located Whether you like mountain biking, bird watching, sailing, relaxing on the beach or sitting in Alberta’s provincial parks and recreation areas. Many more details about these around the campfire, Alberta Parks have a variety of facilities and an infinite supply of Provincial Park campgrounds, as well as group camping, comfort camping and backcountry camping, memory making moments for you. It’s your choice – sweeping mountain vistas, clear Provincial Recreation Area can be found at albertaparks.ca. northern lakes, sunny prairie grasslands, cool shady parklands or swift rivers flowing through the boreal forest. Try a park you haven’t visited yet, or spend a week exploring Activities Amenities Our Vision: Alberta’s parks inspire people to discover, value, protect and enjoy the several parks in a region you’ve been wanting to learn about. Baseball Amphitheatre natural world and the benefits it provides for current and future generations. Beach Boat Launch Good Camping Neighbours Since the 1930s visitors have enjoyed Alberta’s provincial parks for picnicking, beach Camping Boat Rental and water fun, hiking, skiing and many other outdoor activities. Alberta Parks has 476 Part of the camping experience can be meeting new folks in your camping loop. -

Jackfish Lake State of the Watershed Report
Jackfish Lake State of the Watershed Report April 2016 North Saskatchewan Watershed Alliance 202 - 9440 49th St NW Edmonton, AB T6B 2M9 Tel: (587) 525-6820 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non-profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy.. This report was prepared by Jennifer Regier, B.Sc. and David Trew, P.Biol of the North Saskatchewan Watershed Alliance. Cover photo credit: Dara Choy, Stony Plain AB Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2016. Jackfish Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB. for the Jackfish Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/ nswa_publications Jackfish Lake State of the Watershed Report Executive Summary The purpose of this report is to consolidate environmental information on Jackfish Lake and its watershed in an effort to support future planning and management discussions. The report provides perspective on current environmental conditions at the lake relative to regional and historic trends. The report is provided as advice to the Jackfish Lake Management Association (JLMA), Alberta Environment and Parks, and Parkland County. The technical information contained in this document is detailed and addresses many lake and watershed features. -

X X 6 ROCKY 4 Alexo-Saunders X 7MOUNTAIN HOUSE 6 Chambers Creek # 7 Crimson Lake X 10 Eagle Point Priority 5 Priority 3 1 # X Proposed Tourism Node
North Saskatchewan Region: Regional Advisory Council Recommended Recreation Approaches ! ! ! ! ! ! BASCA ! HA ! LOWER AT ! ! ! Approach 1: Priority Recreation Management Planning ! REGION UPP ! ER PEACE REGION ! ! Priority 1 Upper (East) Planning Area ! ! Priority 2 Long Lake Planning Area B ! ! UPPER ! Priority 3 Lower and Center Planning Area ! ! Priority 4 Upper (Central) Planning Area ! PEA ! CE 13 12 ST. PAUL ! Priority 5 Ram Planning Area ! REDWATER Priority 6 GranUdpper (West) Planning Area ! e ! Cache er # ! iv Proposed Public Land Recreation Area ! R th Sa Nor skatchewan ! Approach 2: Upgrades to Existing Parks Priority 2 ! S ! ! A Priority 1 Pigeon Lake A ! S B Priority 2 Long Lake ! K ! C Priority 3 Calhoun Bay ! EDMONTON A ! T D Priority 4 Crimson Lake UPPER ATHABASCA REGION ! 11 C E Priority 5 Fish Lake ! ! H F Priority 6 Goldeye Lake ! ! 10 R E G Priority 7 Red Deer River LLOYDMINSTE ! W ! LEDUC adius DRAYTON Edmonton 100km R ! A Approach 3: Additions of New Lands VALLEY ! N ! Proposed Recreation Area with Consensus # ! ! 2 Bighorn Dam ! CAMROSE ! Priority 1 ! A 5 Jackfish Lake C ! X 9 ! WAINWRIGHT 8 Red Deer River # ! Priority 6 ! ! 9 Buck Lake ! B! a 11 Cooking Lake-Blackfoot # ! ttle ! # ! Ri ! v 12 Bellis Lake ! er C.F.B WAINWRIGHT ! ! ! ! ! ! ! ! 13 Long Lake Priority 4 ! # Proposed Recreation Area with No Consensus # 1 Preachers Point 5 D X! 3 Crescent Falls X X 6 ROCKY 4 Alexo-Saunders X 7MOUNTAIN HOUSE 6 Chambers Creek # 7 Crimson Lake X 10 Eagle Point Priority 5 Priority 3 1 # X Proposed Tourism Node # Bighorn Backcountry Priority 3 RED DEER REGION City, Town # First Nations Reserve !X Métis Settlement # NORDEGG B BANFF F X 4 X Military Reserve R I NATIONAL Provincial Park or Protected Area T 8 E I G National Park S PARK H 3 Green Area C X White Area O L Hydrography U 2 Provincial Highway M B Kilometers I A 0 25 50 100 # Published: December 15, 2014 Information as depicted is subject to change, therefore the Government SOUTH SASKATCHEWAN REGION of Alberta assumes no responsibility for discrepancies at time of use. -

2019-2020 Fisheries Regulation Changes Online Consultation Summary 1
Alberta Environment and Parks, Government of Alberta January 2019 2019-2020 Fisheries Regulation Changes Online Summary URL of document to be assigned by communications Table of Contents Table of Contents ........................................................................................................................... 1 SURVEY ........................................................................................................................................... 3 Approach ...................................................................................................................................... 3 Participation ................................................................................................................................. 4 Response ..................................................................................................................................... 4 RESULTS ......................................................................................................................................... 5 Affiliation of Survey Participants .................................................................................................. 5 Summary of Management Objectives .......................................................................................... 6 Detailed Survey Results ............................................................................................................... 8 ES2 – Jackfish Lake ................................................................................................................ -
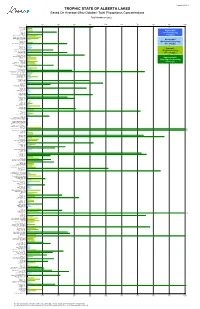
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Fort Chip Meeting Presentation for Website
2008 Monitoring Results Fort Chipewyan, June 9, 2009 Presented by: Fred Kuzmic Wade Gibbons Martin Davies Heather Keith © Hatfield Consultants. All Rights Reserved. 8/12/2009 SLIDE 1 What is RAMP? Initiated in 1997, the Regional Aquatics Monitoring Program (RAMP) is a joint environmental monitoring program that assesses the health of rivers and lakes in the oil sands region. © RAMP. All Rights Reserved. SLIDE 2 1 RAMP Objectives Monitor aquatic environments in the oil sands area; Collect data to better understand the RAMP study area; Compare monitoring data with Environmental Impact Assessment predictions; and Respond to community concerns. © RAMP. All Rights Reserved. SLIDE 3 RAMP Studies Water Quality; Sediment Quality Benthic Invertebrate Communities; Fish Populations; Climate and Water Flow; Acid Sensitive Lakes River Response Networks Reporting and Communications © RAMP. All Rights Reserved. SLIDE 4 2 2008 Monitoring Activities - Athabasca River & Delta Water quality on Athabasca River Fish monitoring – Athabasca-Clearwater rivers, Big Island Lake and Moose (Gardiner) Lake Sediment-dwelling invertebrates and sediment quality – Athabasca Delta Water Flow – Athabasca River upstream and downstream of oil sands industry © RAMP. All Rights Reserved. 8/12/2009 SLIDE 5 Water Quality Monitoring Where do we sample? ±45 stations sampled by RAMP each year (seasonally), in Athabasca River and tributaries; In the delta: monthly sampling at Old Fort by Alberta Environment. Map What do we sample? ~90 different water properties/chemicals (more @ AENV); Nutrients, metals (mercury, arsenic), organic chemicals, ions (salts), oxygen, acidity What are we looking for? Change from natural conditions. © RAMP. All Rights Reserved. 8/12/2009 SLIDE 6 3 © RAMP. All Rights Reserved. -

Trails Parks, Provincial Recreations Areas and Events
David Thompson PARKS The David Thompson Country Tourism Region Country encompasses the municipalities of Caroline, Clearwater County, and Rocky Mountain House. Crimson Lake Ram Falls Rocky Mountain House Landslide Hike Highlights of the area include Abraham Lake, Provincial Park Provincial Park National Historic Site Burnstick Lake, Cow Lake, Crimson Lake, Nordegg and Ya Ha Tinda. While the above parks are the most well-known, David Thompson Please visit our website for more information and Country has over twenty Provincial listings of hotels, restaurants, campgrounds, trails Parks, Provincial Recreations Areas and events. and Wilderness Areas for you to where explore. You can find them listed Alberta Parks Bighorn Backcountry at the link. Listing Link Trails link www.davidthompsoncountry.ca Clearwater River Horse Trails When in David Thompson Country you can find TRAILS more visitor information at: DTC has a variety of trails to explore, from multi use If you’re looking for motorized access for your ATV, Rocky Mountain House Visitor Information Centre trails to motorized and non-motorized specific trails. OHV, or Motorbike there are designated areas for you Monday – Friday, 9 AM – 5 PM Our hiking trails vary in difficulty from short walks to enjoy. One area to explore is the Clearwater Trails Ph: 403-845-5450 suitable for a soft adventure seeker to hikes that will Initiative west of Caroline and the Bighorn area also Toll Free: 1-800-565-3793 challenge the hard-core adventurer. has dedicated motorized trails as well. E-mail: [email protected] Mountain and trail biking are popular There are plenty of areas for horses activities as well, with Nordegg and and we have ranches and outfitters Village of Caroline Office Baseline Mountain being two popular offering guided trail rides if you have Tuesday – Friday, 9 AM – 4 PM destinations. -

Monitoring for Invasive Mussels in Alberta’S Irrigation Infrastructure: 2017 Report
Monitoring for Invasive Mussels in Alberta’s Irrigation Infrastructure: 2017 Report Alberta Agriculture and Forestry Water Quality Section Outlet of Sauder Reservoir January 2018 Introduction and Summary The Government of Alberta (GOA) is committed to protecting the province against aquatic invasive species (AIS), due to their negative ecological and economic effects. Invasive zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena bugensis) are of prominent concern, as these dreissenid mussels attach to any solid submerged surface and rapidly multiply due to their high reproductive rates. They are also very difficult to contain and eradicate once established. Additionally, they are spreading closer to Alberta’s borders. Alberta’s irrigation industry contributes $3.6 billion to the provincial gross domestic product (GDP). Specifically, it contributes about 20% of the provincial agri-food sector GDP on 4.7% of the province’s cultivated land base (Paterson Earth & Water Consulting 2015). Alberta’s irrigation industry includes thirteen irrigation districts that supply water to more than 570,000 ha of farmland through infrastructure valued at $3.6 billion. This infrastructure includes 57 irrigation reservoirs along with 3,491 km of canals and 4,102 km of pipelines (ARD 2014; AF 2017). The irrigation conveyance system provides water to irrigators, municipalities, industries, and wetlands, while the reservoirs support recreational activities such as boating and fishing and provide habitat to fish and waterfowl. Invasive mussels are a concern to the irrigation industry as infestations will have a significant negative effect on water infrastructure and conveyance works due to their ability to completely clog pipelines and damage raw-water treatment systems and intakes. -

Klondike Highway Road Log
Klondike Highway Road Log This 717 km/445 mile road, which is mostly paved, connects Skagway Alaska and Dawson City Yukon , heart of the Klondike. Beginning at Skagway, the road climbs for 14 miles/22.5 km to the 3,292 foot/1003 m summit near the Alaska/Canada border. Between Skagway and the border the road roughly parallels the old White Pass Trail, an alternate to the Chilkoot Trail which ultimately proved more popular as it was the shorter of the two climbs. The only population center along the 114 miles/183 km of connecting routes between Skagway and Whitehorse is the small community of Carcross. At present, there are no services between Skagway and Carcross. The mileage markers along the way indicate the distance from Skagway. Klondike Highwy Map (PDF) Emergency Services Skagway Fire Department-911 Carcross 867- 821-5555 (RCMP) or 867- 821-4444 Medical Emergency Whitehorse Ambulance-911 Carmacks Medical Emergency 867- 863-4444, 867-863-5555 (RCMP) Mayo Medical Emergency 867-996-4444 996-5555 (RCMP) Dawson City Medical Emergency 867- 993-4444; 867-993-5555 (RCMP) Updates on Road Conditions and Construction: http://511.alaska.gov mile 0-Skagway Alaska . Pay attention to the speed limit signs as the police are very serious about the speed limits in Skagway. The reduced speed zone extends to the White Pass Summit mile 14.3/km 23. mile 1.4/km 2.3 Turn-off. On right (for northbound). Follows railroad track .6 mile/1 km to Gold Rush Cemetery where Jefferson Randolph "Soapy" Smith and Frank Reid are buried. -

Alberta Cyanobacteria Beach Monitoring 2010-2013
Alberta Health, Health Protection Alberta Health ALBERTA CYANOBACTERIA BEACH MONITORING 2010–2013 September 2014 Alberta Health, Health Protection Branch Alberta Cyanobacteria Beach Monitoring 2010–2013 September 2014 For more information contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-1-4601-1922-8 (PDF) 2014 Government of Alberta Alberta Health, Health Protection Branch Alberta Cyanobacteria Beach Monitoring 2010–2013 September 2014 EXECUTIVE SUMMARY Harmful blue-green algae (toxic cyanobacteria) blooms in surface water are prevalent in Alberta. The presence of blue-green algae in recreational water causes unpleasant aesthetics. Exposure to some toxin-producing blue-green algae may pose potential health risks to public. There have been increased public awareness and health concerns as a result of increased research over the past 20 years, recent monitoring efforts, as well as the general public becoming educated on the matter. In 2010 and 2011, Alberta Health Services initiated a cyanobacteria monitoring program for shallow water adjacent to beaches and issued public health advisories based on visual inspection. The findings revealed that microcystins (MCYSTs), one group of toxins produced by cyanobacteria, dominate in Alberta’s lakes and reservoirs. In order to inform the residents to safely use public beaches and implement better public health management, Alberta Health and Alberta Health Services along with other governmental departments and public health laboratories conducted the program of Alberta Cyanobacteria Beach Monitoring for Public Health in 2012 and 2013. The objectives of this program are to : 1. establish and maintain an integrated, participatory process for responding to and managing public health issues relating to harmful blue-green algae blooms, 2. -

S T Ony Plain Spruce Gro Ve
CULTURAL PLACES EXPERIENCE TO STAY Q Multicultural Heritage BED & BREAKFASTS Centre Q Rare Gem 5411-51 St Bed, Breakfast & W Parkland Potters’ Guild Private Events 4905-51 Ave 4915-53 Ave E Stony Plain HOTELS/MOTELS & Parkland W Best Western Sunrise Pioneer Museum 5120-43 Ave Inn & Suites PARKLAND COUNTY • SPRUCE GROVE • STONY PLAIN 3101-43 Ave E Motel 6 66 Boulder Blvd STONY PLAIN STONY R Ramada RECREATION TO CITY OF EDMONTON LIMITS (11km) PARKS Inn & Suites & SPORTS 3301-43 Ave T Travelodge Q Heritage Park Pavilion Q Centennial Ball 74 Boulder Blvd T Rotary Wellness Park & 5100-41 Ave Diamonds & Premier Field Volleyball Courts W Lions Park 4210-44 Ave 55 Ave & 49 St 5300-52 St Y Stony Plain BMX Track E W Community Centre Rotary Park 4200-49 Ave 1 CENTURY RD RV PARKS & Horseshoe Pits & Visitor Centre CAMPGROUNDS 5008-51 Ave Q Stony Plain 4815-44 Ave Jubilee Q Outdoor Pool & Park R Shikaoi Park Glenn Hall Q Rotary Spray Park 4905-51 Ave Camp ’n’ Class RV Park Centennial Arena 4107-50 St 5300-52 St 5210-55 Ave T GROVE DR Whispering Y Stony Plain W Lions’ RV Park E High Park Reservoir 2 Waters Park 9 Granite Dr Skateboard Park GROVE DR 49 Ave & Brown St Tennis Courts 3 12 High Park Rd 49 Ave adjacent to CN Tunnel 1 5 R Meridian Heights Q Westridge Curling Club Reservoir Tennis & 5400-52 St Pickleball Courts 4501-43 Ave Photo Credit: Kerri Park Photography OUTDOOR Millgrove Dr Woodhaven Dr SKATING RINKS Y KING ST 7 Q Centennial Memorial WA 2 5300-52 St U École Meridian 3 6 1 WOOD AVE Heights School 4121-43 St 1 2 I 1 Forest Green School PIONEER RD CENTURY RD CALAHOO RD 5206-45 St JENNIFER HEIL GOLF COURSE RD O John Paul II School 2 5801-48 St M McLEOD P Rotary Pond AVE 4815-44 Ave McLEOD AVE 2 McLEOD AVE BOULDER BLVD 3 5 2 TO CITY OF EDMONTON LIMITS (11km) TO JASPER 3 4 2 10 40 AVE 41 AVE 1 28 ST TRANSALTA TRI LEISURE CENTRE 1 4 SOUTH AVE 3 Secondary Highway 779 - FIFTH MERIDIAN 7 44 AVE SOUTH PK RD. -

Decision 25369-D01-2020 Altalink and Transalta DACDA for The
Decision 25369-D01-2020 AltaLink Management Ltd. and TransAlta Corporation Direct Assigned Capital Deferral Account for the Edmonton Region Project December 10, 2020 Alberta Utilities Commission Decision 25369-D01-2020 AltaLink Management Ltd. and TransAlta Corporation Direct Assigned Capital Deferral Account for the Edmonton Region Project Proceeding 25369 December 10, 2020 Published by the: Alberta Utilities Commission Eau Claire Tower 1400, 600 Third Avenue S.W. Calgary, Alberta T2P 0G5 Telephone: 310-4AUC (310-4282 in Alberta) 1-833-511-4AUC (1-833-511-4282 outside Alberta) Email: [email protected] Website: www.auc.ab.ca The Commission may, within 30 days of the date of this decision and without notice, correct typographical, spelling and calculation errors and other similar types of errors and post the corrected decision on its website. Contents 1 Decision summary ................................................................................................................ 1 2 Background .......................................................................................................................... 1 2.1 Project description ........................................................................................................ 1 2.2 Direct assigned capital deferral account application .................................................... 3 2.3 Cost summary ............................................................................................................... 4 3 Prudency assessment of costs .............................................................................................