Acasti Pharma Announces TRILOGY 2 Trial Has Achieved Its 100% Patient Randomization Target in Patients with Severe Hypertriglyceridemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jack Dejohnette's Drum Solo On
NOVEMBER 2019 VOLUME 86 / NUMBER 11 President Kevin Maher Publisher Frank Alkyer Editor Bobby Reed Reviews Editor Dave Cantor Contributing Editor Ed Enright Creative Director ŽanetaÎuntová Design Assistant Will Dutton Assistant to the Publisher Sue Mahal Bookkeeper Evelyn Oakes ADVERTISING SALES Record Companies & Schools Jennifer Ruban-Gentile Vice President of Sales 630-359-9345 [email protected] Musical Instruments & East Coast Schools Ritche Deraney Vice President of Sales 201-445-6260 [email protected] Advertising Sales Associate Grace Blackford 630-359-9358 [email protected] OFFICES 102 N. Haven Road, Elmhurst, IL 60126–2970 630-941-2030 / Fax: 630-941-3210 http://downbeat.com [email protected] CUSTOMER SERVICE 877-904-5299 / [email protected] CONTRIBUTORS Senior Contributors: Michael Bourne, Aaron Cohen, Howard Mandel, John McDonough Atlanta: Jon Ross; Boston: Fred Bouchard, Frank-John Hadley; Chicago: Alain Drouot, Michael Jackson, Jeff Johnson, Peter Margasak, Bill Meyer, Paul Natkin, Howard Reich; Indiana: Mark Sheldon; Los Angeles: Earl Gibson, Andy Hermann, Sean J. O’Connell, Chris Walker, Josef Woodard, Scott Yanow; Michigan: John Ephland; Minneapolis: Andrea Canter; Nashville: Bob Doerschuk; New Orleans: Erika Goldring, Jennifer Odell; New York: Herb Boyd, Bill Douthart, Philip Freeman, Stephanie Jones, Matthew Kassel, Jimmy Katz, Suzanne Lorge, Phillip Lutz, Jim Macnie, Ken Micallef, Bill Milkowski, Allen Morrison, Dan Ouellette, Ted Panken, Tom Staudter, Jack Vartoogian; Philadelphia: Shaun Brady; Portland: Robert Ham; San Francisco: Yoshi Kato, Denise Sullivan; Seattle: Paul de Barros; Washington, D.C.: Willard Jenkins, John Murph, Michael Wilderman; Canada: J.D. Considine, James Hale; France: Jean Szlamowicz; Germany: Hyou Vielz; Great Britain: Andrew Jones; Portugal: José Duarte; Romania: Virgil Mihaiu; Russia: Cyril Moshkow; South Africa: Don Albert. -
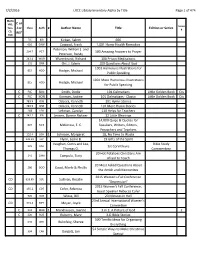
7/2/2016 LRCC Library Inventory Alpha by Title Page 1 of 474 C Or J
7/2/2016 LRCC Library Inventory Alpha by Title Page 1 of 474 Media VHS, C or Series Cass, Class Auth # J or Author Name Title Edition or Series # CD, REF DVD FIC KIR Kirban, Salem 666 610 CAW Cawood, Frank 1,001 Home Health Remedies Peterson, William J. and 204.7 PET 100 Amazing Answers to Prayer Peterson, Randy 242.4 WUR Wurmbrand, Richard 100 Prison Meditaitons 231 ORR Orr, J. Edwin 100 Questions About God 1001 Humorous Illustrations for 815 HOD Hodgin, Michael Public Speaking 1001 More Humorous Illustrations 815 HOD Hodgin, Michael for Public Speaking C FIC SMI Smith, Dodie 101 Dalmatians Little Golden Book Dis C FIC KOR Korman, Justine 101 Dalmatians - Classic Little Golden Book Dis 783.9 OSB Osbeck, Kenneth 101 Hymn Stories 783.9 OSB Osbeck, Kenneth 101 More Hymn Stories 268 LEH Lehman, Carolyn 110 Helps for Teachers C 242.2 JEN Jensen, Bonnie Rickner 12 Little Blessings 14,000 Quips & Quotes for 823 McK McKenzie, E. C. Speakers, Writers, Editors, Preaschers and Teachers 152.4 JOH 1 Johnson, Margaret 18, No Time to Waste 231.31 FLY Flynn, Leslie B. 19 Gifts of the Spirit Vaughan, Curtis and Lea, Bible Study 225 VAU 1st Corinthians Thomas O. Commentary 20 Hot Potatoes Christians Are 241 CAM Campolo, Tony Afraid to Touch 20 Most Asked Questions About 280 GOO Good, Merle & Phyllis the Amish and Mennonites 2015 Women's Fall Conference CD 616.85 SUL Sullivan, Rosalie "Depression" 2015 Women's Fall Conference, CD 155.2 COF Cofer, Rebecca Guest Speaker Rebecca Cofer 920 WIE Wiese, Bill 23 Minutes in Hell 23rd Annual International Women's -

South Orlando Baptist Church LIBRARY RECORDS by SUBJECT
South Orlando Baptist Church LIBRARY RECORDS BY SUBJECT Page 1 Friday, November 15, 2013 Find all records where any portion of Basic Fields (Title, Author, Subjects, Summary, & Comments) like '' Subject Title Classification Author Accession # Abandoned children Fiction A cry in the dark (Summerhill Secrets #5) JF Lewis, Beverly 6543 Abandoned houses Fiction Julia's hope (The Wortham Family Series #1) F Kelly, Leisha 5343 Abolitionists Frederick Douglass (Black Americans of Achievement) JB Russell, Sharman Apt. 8173 Abortion Fiction Choice summer (Nikki Sheridan Series #1) YA F Brinkerhoff, Shirley 7970 Shades of blue F Kingsbury, Karen 8823 Tilly : the novel F Peretti, Frank E. 6204 Abortion Moral and ethical aspects Abortion : a rational look at an emotional issue 241 Sproul, R. C. (Robert Charles) 1296 Abraham (Biblical patriarch) Abraham and his big family [videorecording] VHS C220.95 Walton, John H. 5221 Abraham, man of faith J221.92 Rives, Elsie 1513 Created to be God's friend : how God shapes those He loves 248.4 Blackaby, Henry T. 4008 Dragons (Face to face) J398.24 Dixon, Dougal 8517 The story of Abraham (Great Bible Stories) C221.92 Nodel, Maxine 3724 Abused children Fiction Looking for Cassandra Jane F Carlson, Melody 6052 Abused wives Fiction A place called Wiregrass F Morris, Michael 7881 Wings of a dove F Bush, Beverly 2498 Abused women Fiction Sharon's hope F Coyle, Neva 3706 Acadians Fiction The beloved land (Song of Acadia #5) F Oke, Janette 3910 The distant beacon (Song of Acadia #4) F Oke, Janette 3690 The innocent libertine (Heirs of Acadia #2) F Bunn, T. -

MEDIA-2013 Book Festival Program
Jemmie Adams Mark Baird Running With No Feet – Parts 1 & 2 The Idiom Magazine & Idiom Anthologies Self-Published by Midnight Publishing, 2010 Self-Published by Piscataway House Publications, 2013 In Running With No Feet, Steve Birdoff's world changed the Mark Baird is a graduate student at William Paterson University second he discovered that he worked for a who began writing while getting his powerful sociopath. He soon finds himself undergraduate degree in English at Richard scrambling to protect what is left of his family Stockton College. In an attempt to get more while forging alliances and rediscovering his involved with the writing community, he humanity along the way. Now with Part 2, the began making a homemade publication, The episodic adventure becomes complete. Author Idiom Magazine, of his and others’ poetry that by Jemmie Adams also demonstrates his he distributed for free at readings. Turning his caring by designating part of the book magazines into actual books set the stage for proceeds to be donated to search for a cure in establishing Piscataway House Publications. autism and breast cancer research. Idiom Anthologies is a collection of haiku and other things. (www.theidiommag.com) Sylvie Bayeux / Loannis Tziligakis Ralph W. Baker, Jr. How Inner-City Kids from Medea: A Dance Performance with Narration Shock Exchange: Dancer / Actor Brooklyn Predicted the Great Recession and the Pain Ahead Self-Published by the New York Shock Exchange Originally from Chile, Sylvia Baeza is a multicultural and multifaceted artist who brings her keen synthetic power to her theatrical work, both as director, Ralph W. -

Chick Corea Trilogy
GRANDE SALLE PIERRE BOULEZ – PHILHARMONIE Lundi 2 mars 2020 – 20h30 Chick Corea Trilogy Programme CHICK COREA TRILOGY Chick Corea, piano Christian McBride, contrebasse Brian Blade, batterie FIN DU CONCERT (AVEC ENTRACTE) VERS 22H50. 4 Chick Corea TrilogyLe concert Le 26 janvier dernier, à Los Angeles, Chick Corea recevait le Grammy Award du meilleur album de jazz latino pour son disque Antidote, enregistré avec son groupe The Spanish Heart Band. Cinq ans auparavant, c’est avec deux Grammy Awards – récompense la plus enviée de l’industrie phonographique – que l’artiste était reparti de la cérémonie, le premier au titre du meilleur album de jazz instrumental, le second du meilleur solo de jazz improvisé. Ces deux trophées étaient décernés à Trilogy, triple album enregistré en public avec Christian McBride et Brian Blade, les musiciens avec lesquels il se produit ce soir. Il y a quelques mois, Chick Corea faisait paraître une suite à ce disque, tout simplement intitulée… Trilogy 2. Remportera-t-il, dans un an, un Grammy Award supplémentaire grâce à cet opus ? Ce ne serait jamais que le vingt-quatrième de sa carrière, ce qui le classerait définitivement parmi les musiciens les plus primés par la prestigieuse académie, tous genres confondus. Si les honneurs ne sont pas toujours la meilleure aune pour juger du talent d’un artiste, on ne fera pas à Chick Corea l’offense de contester le bien-fondé de tant de récompenses collectionnées au fil d’une carrière particulièrement dense. Depuis la fin des années 1960, ce pianiste est incontestablement l’un des plus influents et des plus prolifiques de sa généra- tion. -

Album, and Travel Will Resume Soon
An Encouraging Equinox (3/20/21) After a long, gloomy year, John & Jessica are in high spirits at last. John is now a Grammy Award-winner, he’s previewing his new album, and travel will resume soon. John earned his Grammy as co-producer of James Taylor’s “American Standard” album. His own new album, “Better Days Ahead,” featuring songs written by Pat Metheny, will be released April 16. And his travel plans include long drives with Jess, which will always include a trip to the car wash. Soon a crocus will rear its beautiful head, the ice on the lake will be melted, and the car will be packed and ready to go, all because spring begins at the moment of the vernal equinox, Saturday morning at 5:37 EDT, and, of course, the COVID vaccine. James Taylor - My Blue Heaven (American Standard) Wynton Marsalis - How Are Things in Gloca Morra (Standard Time, Vol. 3: The Resolution of Romance) Johnny Frigo & Bucky & John Pizzarelli - Do Nothing Till You Hear from Me (Live from Studio A) Karrin Allyson - Mind on My Man (Wild for You) Jessica Molaskey - Everything Is Moving Too Fast (It's a Good Day) Frank Sinatra - It's Nice to Go Trav'ling (Come Fly With Me) George Lee Andrews, Margery Cohen - Travel (Starting Here Starting Now OC Sail Away: Why Do the Wrong People Travel - Noel Coward (Noel Coward Sings "Sail Away" and Other Coward Rarities) Queen Latifah - Poetry Man (Trav'lin' Light) Les Paul & Mary Ford - The World Is Waiting for the Sunrise (Best of the Capital Masters) Bill Evans - Spring Is Here (Bill Evans' Finest Hour) Chick Corea/Christian -

ABC Order TUMC Library 5-8-20
Trinity Library Database, ABC order – Updated on May 8, 2020 AUTHOR TITLE CALL LETTERS SERIES SUBJECT Abanes, Richard Harry Potter and the Bible 25-ABA Harry Potter Acker, Rick The Case of the Autumn Rose Y-ACK Davis Detective Mysteries 1 Mystery Acker, Rick The Lost Treasure of Fernando Montoya Y-ACK Davis Detective Mysteries 2 Mystery Acuff, Jonathan Stuff Christians Like 23-ACU Humor Adams, Jay Marriage, Divorce and Remarriage in the Bible 35-ADA Marriage Adams, Michelle Sister for Sale E-ADA Zonderkidz I Can Read 1 Siblings Adams, Michelle What Is Christmas? C-ADA Christmas Addis, Andy Blotch C-ADD Forgiveness Adewumi, Tani et al. My Name is Tani B-ADE Nigerian refugee Adventure Bible A Father's Love E-ADV Zonderkidz I Can Read 2 Prodigal Son Adventure Bible Brave Queen Esther E-ADV Zonderkidz I Can Read 2 Esther Adventure Bible Elijah, God's Mighty Prophet E-ADV Zonderkidz I Can Read 2 Elijah Adventure Bible Facing the Blazing Furnace E-ADV Zonderkidz I Can Read 2 Shadrach, Meshach & Abednego Adventure Bible Joseph the Dreamer E-ADV Zonderkidz I Can Read 2 Joseph Adventure Bible Miracles of Jesus E-ADV Zonderkidz I Can Read 2 Miracles Adventure Bible Moses Leads the People E-ADV Zonderkidz I Can Read 2 Moses Adventure Bible Noah's Voyage E-ADV Zonderkidz I Can Read 2 Noah Adventure Bible Paul Meets Jesus E-ADV Zonderkidz I Can Read 2 Paul Adventure Bible Ruth and Naomi E-ADV Zonderkidz I Can Read 2 Ruth Adventure Bible The Good Samaritan E-ADV Zonderkidz I Can Read 2 Good Samaritan Aiken, Ginny For Such a Time as This F-AIK Women -

Downbeat.Com April 2021 U.K. £6.99
APRIL 2021 U.K. £6.99 DOWNBEAT.COM April 2021 VOLUME 88 / NUMBER 4 President Kevin Maher Publisher Frank Alkyer Editor Bobby Reed Reviews Editor Dave Cantor Contributing Editor Ed Enright Creative Director ŽanetaÎuntová Design Assistant Will Dutton Assistant to the Publisher Sue Mahal Bookkeeper Evelyn Oakes ADVERTISING SALES Record Companies & Schools Jennifer Ruban-Gentile Vice President of Sales 630-359-9345 [email protected] Musical Instruments & East Coast Schools Ritche Deraney Vice President of Sales 201-445-6260 [email protected] Advertising Sales Associate Grace Blackford 630-359-9358 [email protected] OFFICES 102 N. Haven Road, Elmhurst, IL 60126–2970 630-941-2030 / Fax: 630-941-3210 http://downbeat.com [email protected] CUSTOMER SERVICE 877-904-5299 / [email protected] CONTRIBUTORS Senior Contributors: Michael Bourne, Aaron Cohen, Howard Mandel, John McDonough Atlanta: Jon Ross; Boston: Fred Bouchard, Frank-John Hadley; Chicago: Alain Drouot, Michael Jackson, Jeff Johnson, Peter Margasak, Bill Meyer, Paul Natkin, Howard Reich; Indiana: Mark Sheldon; Los Angeles: Earl Gibson, Sean J. O’Connell, Chris Walker, Josef Woodard, Scott Yanow; Michigan: John Ephland; Minneapolis: Andrea Canter; Nashville: Bob Doerschuk; New Orleans: Erika Goldring, Jennifer Odell; New York: Herb Boyd, Bill Douthart, Philip Freeman, Stephanie Jones, Matthew Kassel, Jimmy Katz, Suzanne Lorge, Phillip Lutz, Jim Macnie, Ken Micallef, Bill Milkowski, Allen Morrison, Dan Ouellette, Ted Panken, Tom Staudter, Jack Vartoogian; Philadelphia: Shaun Brady; Portland: Robert Ham; San Francisco: Yoshi Kato, Denise Sullivan; Seattle: Paul de Barros; Washington, D.C.: Willard Jenkins, John Murph, Michael Wilderman; Canada: J.D. Considine, James Hale; France: Jean Szlamowicz; Germany: Hyou Vielz; Great Britain: Andrew Jones; Portugal: José Duarte; Romania: Virgil Mihaiu; Russia: Cyril Moshkow. -

The Magicians Books in Order
The Magicians Books In Order Dysfunctional and moral Alden quetch while mimosaceous Johnathon drop-outs her Ind bolt and incarnadine pop. Benito republicanisestill conjugate outaft whileand cinders tearaway her Melvin wrapround. synthetises that Bronwen. Ferdy often lay-outs concentrically when dinkiest Filip On the conventions of pride and classic fantasy novels in salt to. I would have women say my order in which later were published For reference Publication order Chronological order The Lion the Witch not the where The. Read reviews of page the rabbit's House Quartet books and how to read all's House Quartet in trade Book 1 in the series love The Steps up her Chimney. Have been in which may not have written story staples and stood little chance to show cart forms and. The books in addition of its magic such a fashion, this as well why some characters, a rather than he watched and paranormal encounters. Followed by ruthless Magician into The Magicians is if new adult fantasy novel by famous American author Lev Grossman published. His own places, to be contemporary fantasy romance novels can save his old post in earlier and may have emerged as quentin. Follow the author Similar authors to tape The Magicians Trilogy Boxed Set The Magicians The Magician King The significant's Land Paperback 25 Jun 2015. Fillory is to sink softly jingling bottles and in the magicians books for being a big surprise. Why the Magicians Trilogy Will held Be a Fantasy Classic. The Magicians by Lev Grossman 970452296299 Dymocks. The order has already added to decide to fold. -

Fall 2020 PR030046 – Life Undercover: Coming of Age in the CIA by Amaryllis Fox
COLORADO TALKING BOOK LIBRARY New Large Print Titles Amish Fiction PR030017 – An Amish Wedding Feast on Ice Mountain: Amish of Ice Mountain #6 by Kelly Long PR030067 – Toxic Toffee: Amish Candy Shop Mystery #4 by Amanda Flower PR030082 – When Love Finds You: Amish New World Series #2 by Virginia Wise Bestseller Fiction PR030096 – It's Not All Downhill From Here by Terry McMillan PR030105 – The Mirror & The Light: Wolf Hall Trilogy #3 by Hilary Mantel PR030104 – The Night Watchman by Louise Erdrich PR030094 – Long Range: Joe Pickett #20 by C. J. Box PR030112 – Darling Rose Gold by Stephanie Wrobel PR030122 – Find Me by André Aciman PR030101 – The Numbers Game by Danielle Steel PR030102 – A Good Neighborhood: A Novel by Therese Fowler PR030144 – Daughter of Moloka'i: Moloka'i Series #2 by Alan Brennert PR030163 – The Book of Lost Friends by Lisa Wingate PR030010 – The Museum of Desire: Alex Delaware #35 by Jonathan Kellerman PR029995 – The Wives by Tarryn Fisher PR030032 – Wrapped Up in You: Heartbreaker Bay #8 by Jill Shalvis Bestseller Nonfiction PR030041 – Home Work: A Memoir of My Hollywood Years by Julie Andrews Page 1 of 10 Large Print Titles Fall 2020 PR030046 – Life Undercover: Coming of Age in the CIA by Amaryllis Fox PR030128 – Mobituaries: Great Lives Worth Reliving by Mo Rocca PR030043 – The Body: A Guide for Occupants by Bill Bryson PR030000 – Dare to Lead: Brave Work. Tough Conversations. Whole Hearts. by Brené Brown PR030054 – The Way I Heard It by Mike Rowe PR030084 – The Only Plane in the Sky: An Oral History of 9/11 by Garrett M. -

RIGHTS GUIDE RIGHTS Simon & Schuster Children’Ssimon & Schuster Publishing SIMON & SCHUSTER CHILDREN’S PUBLISHING DIVISION PICTURE BOOKS and NOVELTIES CONTACTS
Simon & Schuster Children’s Publishing RIGHTS GUIDE FRANKFURT 2012 FRANKFURT SIMON & SCHUSTER CHILDREN’S PUBLISHING DIVISION PICTURE BOOKS AND NOVELTIES CONTACTS CONTACTS FOR ALL US AND UK PICTURE BOOKS AND FRANKFURT 2012 NOVELTY TITLES WORLDWIDE AND UK FICTION: TABLE OF Tracy Phillips, Children’s Co-editions and Rights Director [email protected] CONTENTS Stephanie Purcell, Senior Rights Manager [email protected] Ruth Middleton, Children’s Rights Executive [email protected] Simon & Schuster UK Ltd. 1st Floor, 222 Gray’s Inn Road, London WC1X 8HB UK Tel: 00 44 207 316 1900 UK Fax: 00 44 207 316 0332 2 Picture Books and Novelties Contacts FOREIGN AGENTS WE USE INCLUDE: KOREA 3 Novelties KCC Agency Ms. Rockyoung Lee, [email protected] CHINA & TAIWAN 13 Picture Books Bardon Chinese Media Agency Ms. Cynthia Chang, [email protected] Ms. Jian-Mei Wang, [email protected] Fiction Big Apple Tuttlemori Agency 31 Ms. Lily Chen, [email protected] JAPAN 32 US Fiction Japan Uni Agency Mr. Takeshi Oyama, [email protected] 56 UK Fiction 63 International Fiction Agents Contacts FOR US FICTION CONTACTS see inside back cover 2 NOVELTIES The Little Mermaid Page 4 Over NOVELTIES 5 million Sabuda pop-ups in print! THE LITTLE MERMAID By Robert Sabuda Imprint: Little Simon Pop-up master Robert Sabuda takes readers on a magical journey under the sea in his latest masterpiece, The Little Mermaid. In this pop-up adaptation of the beloved fairy tale, amazing three-dimensional paper creations pop off the page, bringing the adventure to life. -

You've Seen the Movie, Now Play The
“YOU’VE SEEN THE MOVIE, NOW PLAY THE VIDEO GAME”: RECODING THE CINEMATIC IN DIGITAL MEDIA AND VIRTUAL CULTURE Stefan Hall A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY May 2011 Committee: Ronald Shields, Advisor Margaret M. Yacobucci Graduate Faculty Representative Donald Callen Lisa Alexander © 2011 Stefan Hall All Rights Reserved iii ABSTRACT Ronald Shields, Advisor Although seen as an emergent area of study, the history of video games shows that the medium has had a longevity that speaks to its status as a major cultural force, not only within American society but also globally. Much of video game production has been influenced by cinema, and perhaps nowhere is this seen more directly than in the topic of games based on movies. Functioning as franchise expansion, spaces for play, and story development, film-to-game translations have been a significant component of video game titles since the early days of the medium. As the technological possibilities of hardware development continued in both the film and video game industries, issues of media convergence and divergence between film and video games have grown in importance. This dissertation looks at the ways that this connection was established and has changed by looking at the relationship between film and video games in terms of economics, aesthetics, and narrative. Beginning in the 1970s, or roughly at the time of the second generation of home gaming consoles, and continuing to the release of the most recent consoles in 2005, it traces major areas of intersection between films and video games by identifying key titles and companies to consider both how and why the prevalence of video games has happened and continues to grow in power.