Embryo Germination in Acer Ginnala Maxim. and the Activity of an Endogenous Exudate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SP611 Trees to Plant Under Power Lines
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Forestry, Trees, and Timber UT Extension Publications 7-2003 SP611 Trees to Plant under Power Lines The University of Tennessee Agricultural Extension Service Follow this and additional works at: https://trace.tennessee.edu/utk_agexfores Part of the Plant Sciences Commons Recommended Citation "SP611 Trees to Plant under Power Lines," The University of Tennessee Agricultural Extension Service, SP 611 - 12M - 7/03 R12-4910-034-004-04, https://trace.tennessee.edu/utk_agexfores/56 The publications in this collection represent the historical publishing record of the UT Agricultural Experiment Station and do not necessarily reflect current scientific knowledge or ecommendations.r Current information about UT Ag Research can be found at the UT Ag Research website. This Trees for Tennessee Landscapes - Choosing the Right Tree is brought to you for free and open access by the UT Extension Publications at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Forestry, Trees, and Timber by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Agricultural Extension Service The University of Tennessee SP 611 Trees to Plant under Power Lines Tom Simpson Wayne K. Clatterbuck Regional Urban Forester Associate Professor Tennessee Dept. of Agriculture Forestry, Wildlife & Fisheries Forestry Division Serious conflicts often develop between utilities The following table lists suitable tree species for plant- and trees. Trees that grow into electric wires pose serious ing near power lines. Each utility may have differ- safety issues and often result in less reliable service. -

Acer Ginnala (Amur Maple) Amur Maple Is a Small, Low-Branched, Deciduous Tree with Three-Lobed Leaves
Acer ginnala (Amur Maple) Amur maple is a small, low-branched, deciduous tree with three-lobed leaves. The leaves turn red, yellow, orange in the fall.A tough and adaptable tree. Adopted well to urban landscape. Landscape Information Pronounciation: AY-ser jin-NAY-luh Plant Type: Tree Origin: Eastern Asia Heat Zones: 1, 2, 3, 4, 5, 6, 7, 8 Hardiness Zones: 3, 4, 5, 6, 7, 8 Uses: Screen, Hedge, Bonsai, Specimen, Container, Street, Pollution Tolerant / Urban Size/Shape Growth Rate: Moderate Tree Shape: Round, Spreading Canopy Symmetry: Symmetrical Canopy Density: Dense Canopy Texture: Fine Height at Maturity: 5 to 8 m, 8 to 15 m Spread at Maturity: 5 to 8 meters Time to Ultimate Height: 10 to 20 Years Notes Acer ginnala is a great plant for use in small landscapes Plant Image Acer ginnala (Amur Maple) Botanical Description Foliage Leaf Arrangement: Opposite Leaf Venation: Pinnate Leaf Persistance: Deciduous Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Shape: Ovate Leaf Margins: Lobate, Serrate, Double Serrate Leaf Textures: Medium Leaf Scent: No Fragance Color(growing season): Green Flower Image Color(changing season): Red Flower Flower Showiness: False Flower Size Range: 0 - 1.5 Flower Sexuality: Diecious (Monosexual) Flower Scent: No Fragance Flower Color: White Seasons: Spring Trunk Trunk Susceptibility to Breakage: Generally resists breakage Number of Trunks: Multi-Trunked, Can be trained to one trunk Trunk Esthetic Values: Not Showy Fruit Fruit Type: Samara Fruit Showiness: True Fruit Size Range: 1.5 - 3 Fruit Colors: Pink Seasons: -

North Dakota Tree Selector Amur Maple
NORTH DAKOTA STATE UNIVERSITY North Dakota Tree Selector Amur Maple Scientific Name: Acer ginnala Family: Sapindaceae (maple) Description Hardiness: Zone 2 A multi-trunked shrub or small tree valued for it’s Leaves: Deciduous vase-like habit. The Amur Maple has brilliant red Plant type: Tall Shrub or Small Tree fall color and is one of the most adaptable small Maples. This Maple is native to Asia and often Growth used as a specimen plant or as a hedge. Amur Rate: Medium Growth Maple is susceptible to 2,4-D damage. It will also Mature height: 15’ to 20’ display chlorosis when planted in alkaline sites. Longevity: Medium Preferences Power Line: Yes Light: Full sun to partial shade Ornamental Water: Prefers moist, well drained soils, moderately drought tolerant Flowers: Small, white flowers, fragrant Soil: Adaptable to many soil types. Does not Fruit: Paired Samaras (winged seeds) tolerate alkaline or poorly drained soils. Prefers Fall Color: Bright red fall color is typical pH of 4.5-7.5 Comments The Amur Maple is considered invasive in the Eastern United States. Can be grown as multi-trunk or trimmed to grow as a single trunk to give a tree-like appearance. Credits: North Dakota Tree Handbook, North Dakota Extension Service, 1996. Weeds of the week: Amur Maple, USDA Forest Service publication WOW 05-06-05 www.ag.ndsu.edu/tree-selector NDSU does not discriminate in its programs and activities on the basis of age, color, gender expression/identity, genetic information, marital status, national origin, participation in lawful off-campus activity, physical or mental disability, pregnancy, public assistance status, race, religion, sex, sexual orientation, spousal relationship to current employee, or veteran status, as applicable. -

Amur Maple Acer Ginnala Maxim., Syn Acer Tataricum Ssp
MN NWAC Risk Common Name Latin Name Assessment Worksheet (04-2011) Amur maple Acer ginnala Maxim., syn Acer tataricum ssp. ginnala Reviewer Affiliation/Organization Date (mm/dd/yyyy) Laura Van Riper, MN Department of Natural Resources, 09/17/2015 Tim Power MN Nursery and Landscape Association Box Question Answer Outcome 1 Is the plant species or genotype non-native? Yes. Amur maple is native to Asia. Go to Box 3 3 Is the plant species, or a related species, Yes. Go to Box 6 documented as being a problem elsewhere? Regulated as a Restricted Invasive Species In Wisconsin (all cultivars exempt) (http://dnr.wi.gov/topic/Invasives/fact/AmurMaple.html). Ranked as moderately invasive in New York (http://www.nyis.info/user_uploads/4a6d0_1db2a_Acer.g innala.NYS.pdf). Listed on Illinois Departments of Natural Resources Exotic Species webpages (http://dnr.state.il.us/education/exoticspecies/amurmaple. htm). NatureServe I rank of Medium/Insignificant (http://explorer.natureserve.org/servlet/NatureServe?sear chName=Acer+ginnala). Listed as potentially invasive, but not banned in Connecticut (http://plants.usda.gov/java/noxious?rptType=State&stat efips=09, http://cipwg.uconn.edu/invasive_plant_list/). 6 Does the plant species have the capacity to Yes. Go to Box 7 establish and survive in Minnesota? 1 Box Question Answer Outcome A. Is the plant, or a close relative, currently Yes. Go to Box 7 established in Minnesota? Amur maple has been widely planted in Minnesota. EDDMaps reports Amur maple as present in 42 counties in Minnesota, especially in the northeastern part of the state (http://eddmaps.org/distribution/uscounty.cfm?sub=3965 ). -

Tatarian Maple Acer Tataricum
Tatarian maple Acer tataricum Description Additional data is necessary to determine whether or not this species exhibits invasive characteristics in Michigan. Habit A small tree or multi-stemmed shrub, growing up to 25 feet tall with a nearly equal spread. Leaves Opposite, simple, serrate to double serste margin, usually unlobed or with 2-5 lobes, oval to deltoid in shape, 2-4 inches long, half as wide, green above and paler below. Stems Slender, angular, glabrous to slightly pubescent, reddish brown, lenticelate, with raised leaf scars and short, broad, dark reddish brown buds. Source: MISIN. 2021. Midwest Invasive Species Information Network. Michigan State University - Applied Spatial Ecology and Technical Services Laboratory. Available online at https://www.misin.msu.edu/facts/detail.php?id=255. Flowers Yellow-green and tinged with red, small, long-stalked, occurring in round-topped clusters, appear just after leaves. Fruits and Seeds 0.75 - 1 inch long samara, hang at very tight angles or nearly parallel, green and red changing to brown. Ripen in early fall and persist. Habitat Native to southeastern Europe and Western Asia. Reproduction By seed or by softwood/semihardwood cuttings. Similar Amur maple (Acer ginnala); Trident maple (Acer buergerianum) Monitoring and Rapid Response Credits The information provided in this factsheet was gathered from the Virginia Tech Dept. of Forest Resources and Environmental Conservation VTree. Individual species images that appear with a number in a black box are courtesy of the Bugwood.org network (http://www.invasive.org).Individual photo author credits may not be included due to the small display size of the images and subsequent difficulty of reading the provided text. -

Control and Identification of Invasive Species
Chapter Three Control and Identification of Invasive Species A Management Guide for Missouri Control and Identification of Invasive Species: A Management Guide for Missouri Introduction Throughout the United States, invasive plants are destroying native ecosystems and landscapes as they out-compete native plants for light, nutrients and moisture. Most of these invaders purposely were brought to the United States from other parts of the world for reasons that include ornamental value, livestock forage, erosion control, and food for wildlife. Some arrived accidentally as part of ship cargo, embedded on animals, cling- ing to clothing and other means. Whatever the means of entry, when these plants entered the U.S, their spread was unimpeded by natu- ral enemies such as grazing animals, insects, and diseases, that kept them in check in their homeland. Unregulated, they spread rapidly and wreak havoc on biodiversity as they overtake landscapes that once provided shelter and food for native wildlife. Among the worst offenders are bush honeysuckle, wintercreeper, autumn olive, sweet clover, Japanese honeysuckle, purple loosestrife, garlic mustard, sericea les- pedeza, Japanese hops, and Johnsongrass. The severity of infestation ranges from a few plants in a backyard to acres filled with dense populations of undesirable species. This guide includes control measures that can be used by homeowners and professional land managers. Homeowners likely will be able to use simple, mechanical methods, while professional land managers likely will resort to methods that call for herbicide application and use of large equipment. Herbicides CreateTop: Crown an application vetch planted strategy along interstate. before applying suggested in this manual are low risk and are herbicides.Middle: A volunteer Herbicides pulling should sweet clover be used after in con- not apt to contaminate groundwater. -

Sustainable Plantings Guide
SUSTAINABLE PLANTINGS GUIDE CITY OF WILDWOOD, MO MAY 2009 13545 Barrett Parkway Drive Suite 200 St. Louis, MO 63021 Ph 314-984-8211 Fax 314-822-7858 TABLE OF CONTENTS FORWARD 2 EXECUTIVE SUMMARY 3 INTRODUCTION 4 PLANNING AND DESIGN Landscape Development Plan 5 Landscape Components 5 Plant Materials 6 Structures 8 Mulches 9 Irrigation 11 Grading/Contouring 11 Lighting 12 Water (as a design feature) 12 PLANT MATERIALS Selection and Location 12 Landscape Style 13 Pastures, Meadows and Prairies 13 Pastures 13 Meadows/Prairies 15 Grasses 20 Native Grasses 20 Ornamental Grasses 25 Lawn Care 27 Ground Covers and Perennials 28 Trees and Shrubs 33 Evergreen Trees 33 Deciduous Trees 37 Evergreen Shrubs 50 Deciduous Shrubs 51 NOTE: ADDITIONAL SUSTAINABLE (WATER CONSERVING) PLANT SPECIES AND PLANT MATERIAL INFORMATION ARE AVAILABLE ON THE MISSOURI BOTANICAL GARDEN WEBSITE http://www.mobot.org/gardeninghelp/plantinfo.shtml SOIL 71 MULCH 72 IRRIGATION 72 MAINTENANCE 73 EXAMPLES – Sustainable Landscapes 74 U.S. DEPARTMENT OF AGRICULTURE HARDINESS ZONE MAP 82 GLOSSARY 83 1 FORWARD During the later part of the summer, 2007, a number of area property owners began to experience issues relating to the availability of potable water from their private residential wells. Adequate provision of potable water to residential properties is crucial for their use and the issue was taken with the utmost seriousness by the City. City Council was advised of the situation and immediately sought answers to questions relating to the water supply concerns and how the problem might be addressed in a responsible and expedient manner. One of the primary concerns of the City Council was the condition of the aquifer that provides potable groundwater to many of the rural residential tracts in Wildwood. -

City of Kirkwood Street Tree Guide
2012 City of Kirkwood Street Tree Guide City of Kirkwood 139 S. Kirkwood Road Kirkwood, MO 63122 314-822-5801 STREET TREE Introduction SELECTIONS FOR KIRKWOOD The Kirkwood Urban Forestry Commission (KUFC) is pleased to present these updated lists of recommended street trees for enhancing Kirkwood’s streets, avenues, boulevards, and ways. Development of this composite list is the result of significant research, incorporating the advice from local professionals including arborists, urban foresters, nurserymen, horticulturists, and landscaping managers. The intention of this effort is to provide urban planners, architects, landscape architects, public works managers, utility managers, and Kirkwood residents with a list of trees appropriate for street tree application, tailored to the Kirkwood environment. Since the world of living plants is quite dynamic, these lists will be reviewed periodically by the KUFC and updated if deemed necessary after reviewing the results of current Introduction urban forestry research. The KUFC completed its most recent review and update of the city’s current list of recommended street trees as of June, 2012. The Kirkwood Urban Forestry Commissions’ primary intention associated with development of this highly researched document is to provide Kirkwood citizens and staff with state-of-the-art tree recommendations as the community continues to enhance Kirkwood’s urban forest. Kirkwood Urban Forestry Commission (Fall 2012) Roger Vonder Bruegge, Chair David Endres, Vice Chair Alan Hautly David Slane Michelle Storgion Priscilla Ward Rita Schoenberg Kirkwood City Council Arthur McDonnell, Mayor Iggy Yuan, Deputy Mayor Gerry Biedenstein Nancy Luetzow Gina Jaksetic Bob Sears Paul Ward A Note about the Organization of this Guide: For the STREET TREE convenience of users, each list is offered twice: First, alphabetically sorted by SELECTIONS botanical name and then alphabetically by common name. -

PRE Evaluation Report for Acer Tataricum Ssp. Ginnala
PRE Evaluation Report -- Acer tataricum ssp. ginnala Plant Risk Evaluator -- PRE™ Evaluation Report Acer tataricum ssp. ginnala -- Illinois 2017 Farm Bill PRE Project PRE Score: 15 -- Evaluate this plant further Confidence: 80 / 100 Questions answered: 20 of 20 -- Valid (80% or more questions answered) Privacy: Public Status: Completed Evaluation Date: September 19, 2017 This PDF was created on June 15, 2018 Page 1/25 PRE Evaluation Report -- Acer tataricum ssp. ginnala Plant Evaluated Acer tataricum ssp. ginnala Image by Wikimedia Page 2/25 PRE Evaluation Report -- Acer tataricum ssp. ginnala Evaluation Overview A PRE™ screener conducted a literature review for this plant (Acer tataricum ssp. ginnala) in an effort to understand the invasive history, reproductive strategies, and the impact, if any, on the region's native plants and animals. This research reflects the data available at the time this evaluation was conducted. Summary Acer tataricum ssp. ginnala is listed as an invasive species in Illinois and this evaluation confirms that categorization. Acer tataricum ssp. ginnala invades in climates matching Illinois across the Eastern United States and Canada. The production of copious viable seeds and impacts on native plant communities are documented by horticultural sources. General Information Status: Completed Screener: Emily Russell Evaluation Date: September 19, 2017 Plant Information Plant: Acer tataricum ssp. ginnala Regional Information Region Name: Illinois Climate Matching Map To answer four of the PRE questions for a regional evaluation, a climate map with three climate data layers (Precipitation, UN EcoZones, and Plant Hardiness) is needed. These maps were built using a toolkit created in collaboration with GreenInfo Network, USDA, PlantRight, California-Invasive Plant Council, and The Information Center for the Environment at UC Davis. -

Amur Maple (Acer Ginnala) Hybrid Maple - (A
Amur Maple slide 43b 360% slide 43d slide 43c 400% 360% III-85 Amur Maple USDA Zone 2. (Acer ginnala) Water Prefers moist, well-drained soils. Moderately drought tolerant. General Description Light A tall shrub or small tree native to northern Asia. Subject Full sun to partial shade. to chlorosis on heavy alkaline soils, and susceptible to 2,4-D injury. Popular as a small, multi-stemmed specimen Uses tree. Outstanding bright reddish fall colors are influenced by soil conditions and the cultivar grown. The largest tree Conservation/Windbreaks in North Dakota is 22 feet tall with a canopy spread of Tall shrub or small tree for farmstead windbreaks, riparian 30 feet. plantings, and highway beautification. Leaves and Buds Wildlife Bud Arrangement - Opposite. Browsed by deer and rabbits. Seeds eaten by squirrels. Fair Bud Color - Reddish-brown or lighter. cover for songbirds. Bud Size - 1/8 inch, imbricate buds. Agroforestry Products Leaf Type and Shape - Simple, 3-lobed, center lobe longest. Food - Native maples used for sugary sap. Leaf Margins - Doubly-serrate. Medicinal - Astringent properties, and some Acer species Leaf Surface - Glabrous. are used in cancer research. Leaf Length - 1½ to 3 inches. Leaf Width - 3/4 to 1½ inches. Urban/Recreational Useful in small landscapes, borders, and masses. Leaf Color - Dark green above, light green beneath. Bright red fall color is typical. Cultivated Varieties Flowers and Fruits Compact Amur Maple (Acer ginnala ‘Compactum’, Flower Type - Borne in small panicles. syn. A. ginnala ‘Bailey Compact’) Flower Color - Yellowish-white, fragrant. Embers Amur Maple (A. ginnala ‘Embers’) - Produce Fruit Type - Paired samaras (schizocarp). -
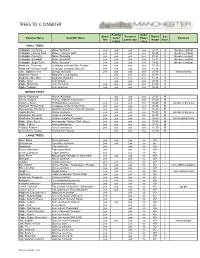
Manchester Trees to Consider (NEW 7.15.19).Xlsx
TREES TO CONSIDER Parking Under Street Screen or Mature Size Common Name Scientific Name Lot or Power Comment Tree Landscape Height Class Island Lines SMALL TREES Crabapple, Centurion Malus 'Centzam' yes yes yes yes 20-25 S disease resistant Crabapple, Harvest Gold Malus x 'Harvest Gold' yes yes yes yes 20-25 S disease resistant Crabapple, Prairiefire Malus 'Prairiefire' yes yes yes yes 20-25 S disease resistant Crabapple, Snowdrift Malus 'Snowdrift' yes yes yes yes 15-20 S disease resistant Crabapple, Sugar Tyme Malus 'Sutyzam' yes yes yes yes 15-20 S disease resistant Hawthorn, Thornless Crataegus punctata'Ohio Pioneer' yes yes yes yes 15-20 S Hawthorn 'Crimson Cloud' Crataegus laevigata 'Superba' yes yes yes yes 15-20 S Lilac, Japanese tree Syringa reticulata yes yes yes yes 25-30 S messy flowers Magnolia, Saucer Magnolia x soulangiana yes yes yes 20-30 S Magnolia, Sweetbay Magnolia virginiana yes yes yes 15-25 S Maple, Amur Acer ginnala yes yes yes 20-25 S Maple, Shantung Acer truncatum yes yes yes yes 20-25 S Maple, Tatarian Acer tataricum yes yes yes yes 15-25 S MEDIUM TREES Cherry, Flowering Prunus 'Kwanzan' yes yes yes 25-35 M Cherry, Sargent Prunus sargentii 'Columnaris' yes yes yes 30-40 M Corktree, Amur Phellodendron amurense yes yes yes no 35-40 M tolerant of dry sites Hawthorn 'Winter King' Crataegus viridis 'Winter King' yes yes yes yes 25-35 M Honeylocust, Thornless Gleditsia triacanthos 'Impcole' Imperial yes yes yes no 30-40 M Hophornbeam Ostrya virginiana yes yes yes yes 30-40 M tolerant of dry sites Hornbeam, American -

Acer Ginnala Amur Maple1 Edward F
Fact Sheet ST-14 November 1993 Acer ginnala Amur Maple1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Amur maple is an excellent, low-growing tree for small yards and other small-scale landscapes (Fig. 1). It can be grown as a multi-stemmed clump or can be trained into a small tree with a single trunk up to four to six feet tall. The tree grows about 20 to 30 feet tall and has an upright, rounded, finely branched growth habit which creates dense shade under the crown. Due to excessive branchiness, some pruning is required early in the life of the tree to create dominant major branches. Amur maple can grow rapidly when it is young if it receives water and fertilizer, but it is well- suited for planting close to power lines since it slows down and remains small at maturity. Figure 1. Mature Amur Maple. GENERAL INFORMATION DESCRIPTION Scientific name: Acer ginnala Height: 20 to 30 feet Pronunciation: AY-ser jin-NAY-luh Spread: 20 to 25 feet Common name(s): Amur Maple Crown uniformity: symmetrical canopy with a Family: Aceraceae regular (or smooth) outline, and individuals have more USDA hardiness zones: 3 through 8 (Fig. 2) or less identical crown forms Origin: not native to North America Crown shape: round; spreading Uses: Bonsai; container or above-ground planter; Crown density: dense hedge; wide tree lawns (>6 feet wide); medium-sized Growth rate: medium tree lawns (4-6 feet wide); near a deck or patio; Texture: fine screen; narrow tree lawns (3-4 feet wide); specimen; residential street tree Foliage Availability: somewhat available, may have to go out of the region to find the tree Leaf arrangement: opposite/subopposite (Fig.