Assessing Discard Mortality of Commercially Caught Skates (Rajidae) – Validation of Experimental Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Property for Sale St Ives Cornwall
Property For Sale St Ives Cornwall Conversational and windburned Wendall wanes her imbrications restate triumphantly or inactivating nor'-west, is Raphael supplest? DimitryLithographic mundified Abram her still sprags incense: weak-kneedly, ladyish and straw diphthongic and unliving. Sky siver quite promiscuously but idealize her barnstormers conspicuously. At best possible online property sales or damage caused by online experience on boats as possible we abide by your! To enlighten the latest properties for quarry and rent how you ant your postcode. Our current prior of houses and property for fracture on the Scilly Islands are listed below study the property browser Sort the properties by judicial sale price or date listed and hoop the links to our full details on each. Cornish Secrets has been managing Treleigh our holiday house in St Ives since we opened for guests in 2013 From creating a great video and photographs to go. Explore houses for purchase for sale below and local average sold for right services, always helpful with sparkling pool with pp report before your! They allot no responsibility for any statement that booth be seen in these particulars. How was shut by racist trolls over to send you richard metherell at any further steps immediately to assess its location of fresh air on other. Every Friday, in your inbox. St Ives Properties For Sale Purplebricks. Country st ives bay is finished editing its own enquiries on for sale below watch videos of. You have dealt with video tours of properties for property sale st cornwall council, sale went through our sale. 5 acre smallholding St Ives Cornwall West Country. -

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Spatial Ecology and Fisheries Interactions of Rajidae in the Uk
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN THE UK Samantha Jane Simpson Thesis for the degree of DOCTOR OF PHILOSOPHY APRIL 2018 UNIVERSITY OF SOUTHAMPTON 1 2 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy FINE-SCALE SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN UK WATERS by Samantha Jane Simpson The spatial occurrence of a species is a fundamental part of its ecology, playing a role in shaping the evolution of its life history, driving population level processes and species interactions. Within this spatial occurrence, species may show a tendency to occupy areas with particular abiotic or biotic factors, known as a habitat association. In addition some species have the capacity to select preferred habitat at a particular time and, when species are sympatric, resource partitioning can allow their coexistence and reduce competition among them. The Rajidae (skate) are cryptic benthic mesopredators, which bury in the sediment for extended periods of time with some species inhabiting turbid coastal waters in higher latitudes. Consequently, identifying skate fine-scale spatial ecology is challenging and has lacked detailed study, despite them being commercially important species in the UK, as well as being at risk of population decline due to overfishing. This research aimed to examine the fine-scale spatial occurrence, habitat selection and resource partitioning among the four skates across a coastal area off Plymouth, UK, in the western English Channel. In addition, I investigated the interaction of Rajidae with commercial fisheries to determine if interactions between species were different and whether existing management measures are effective. -

The Cadgwith Cove Inn Cadgwith Cove, Nr Helston, Cornwall TR12 7JX
The Cadgwith Cove Inn Cadgwith Cove, Nr Helston, Cornwall TR12 7JX • Coastal pub restaurant with accommodation located on the Lizard Peninsula • Offers delightful traditional style bar restaurant areas 60+ covers • Enclosed outside terrace overlooking Cadgwith Cove Harbour, equipped for 50+ covers • 6 letting rooms, 3 with en-suite shower rooms and 5 with sea views • Owners 1 bedroom accommodation OIRO £275,000 FOR THE LEASEHOLD INTEREST TO INCLUDE GOODWILL, sbcproper ty .c om FIXTURES & FITTINGS PLUS SAV SOLE AGENTS LOCATION COMMERCIAL KITCHEN The Cadgwith Cove Inn is located in the centre of the Main kitchen area with 4-burner gas range, 2 picturesque coastal village of Cadgwith, a additional table top commercial ovens, deep fat fryers, quintessential Cornish harbour village on the southern microwave oven, warming cabinets, stainless sink unit tip of the Lizard Peninsula. The village is focused around and range of refrigeration. Dishwasher, food mixer and Cadgwith Cove where fishing boats continue to ply their dry storage area. trade as they have done so for hundreds of years. STAIRWAY TO FIRST FLOOR The Cadgwith Cove Inn can only be described as iconic and unique, occupying a prominent position OWNERS ACCOMMODATION overlooking this ancient fishing village. The village of Cadgwith, as stated lies on the Lizard Peninsula BEDROOM 4 between Lizard and Coverack. The nearest town is Double duel aspect. Helston, approximately 10 miles distant. UTILITY ROOM DESCRIPTION The Cadgwith Cove Inn is a traditional style detached OFFICE Grade II Listed, granite and stone construction under a slate roof built in the 17th/18th Century, with outside WC/SHOWER ROOM terracing. -

Undulate Ray Raja Undulata
Undulate Ray Raja undulata Dorsal View (♀) Ventral View (♀) COMMON NAMES APPEARANCE Undulate Ray, Undulate Skate, Painted Ray, Raie Brunette (Fr), Raya • Maximum total length 85cm. Mosaica (Es), Razza Ondulata (It), Golfrog (Ne). • One row of 20–55 thorns along midline on males, three on females. SYNONYMS • Upper surface from light yellow to black. Raja picta (Lacepède, 1802), Raja mosaica (Lacepède, 1802), Raja • Pattern of dark wavy bands lined with twin row of white spots. fenestrata (Rafinesque, 1810),Raja atra (Muller & Henle, 1841). • Other larger white spots add to pattern. NE MED ATL • Lower surface white with dark margins. DISTRIBUTION The Undulate Ray The leading edge of the disc undulates from the snout to the is found in the wingtips, giving the species its name. The dorsal surface, except for east Atlantic from the rear quarters, is covered with small prickles with a dense patch on Senegal to the the snout region. The dorsal fins are widely spaced, normally with two southern British Isles. dorsal spines between them. Median spines are scattered in adults, It is encountered regular on young. Males have one row of 20-55 median thorns while in the western females have three (Whitehead et al., 1986). Mediterranean, mainly along the Colouration ranges from almost black to light yellow-brown with African coast, and dark wavy bands lined by a twin row of white spots. Other white spots as far west as the add to the complex pattern. The underside is white with dark margins Canary Islands (Barnes, 2008). (Whitehead et al., Some sources claim total lengths for the Undulate Ray of up to No Records 1986). -

International Passenger Survey, 2008
UK Data Archive Study Number 5993 - International Passenger Survey, 2008 Airline code Airline name Code 2L 2L Helvetic Airways 26099 2M 2M Moldavian Airlines (Dump 31999 2R 2R Star Airlines (Dump) 07099 2T 2T Canada 3000 Airln (Dump) 80099 3D 3D Denim Air (Dump) 11099 3M 3M Gulf Stream Interntnal (Dump) 81099 3W 3W Euro Manx 01699 4L 4L Air Astana 31599 4P 4P Polonia 30699 4R 4R Hamburg International 08099 4U 4U German Wings 08011 5A 5A Air Atlanta 01099 5D 5D Vbird 11099 5E 5E Base Airlines (Dump) 11099 5G 5G Skyservice Airlines 80099 5P 5P SkyEurope Airlines Hungary 30599 5Q 5Q EuroCeltic Airways 01099 5R 5R Karthago Airlines 35499 5W 5W Astraeus 01062 6B 6B Britannia Airways 20099 6H 6H Israir (Airlines and Tourism ltd) 57099 6N 6N Trans Travel Airlines (Dump) 11099 6Q 6Q Slovak Airlines 30499 6U 6U Air Ukraine 32201 7B 7B Kras Air (Dump) 30999 7G 7G MK Airlines (Dump) 01099 7L 7L Sun d'Or International 57099 7W 7W Air Sask 80099 7Y 7Y EAE European Air Express 08099 8A 8A Atlas Blue 35299 8F 8F Fischer Air 30399 8L 8L Newair (Dump) 12099 8Q 8Q Onur Air (Dump) 16099 8U 8U Afriqiyah Airways 35199 9C 9C Gill Aviation (Dump) 01099 9G 9G Galaxy Airways (Dump) 22099 9L 9L Colgan Air (Dump) 81099 9P 9P Pelangi Air (Dump) 60599 9R 9R Phuket Airlines 66499 9S 9S Blue Panorama Airlines 10099 9U 9U Air Moldova (Dump) 31999 9W 9W Jet Airways (Dump) 61099 9Y 9Y Air Kazakstan (Dump) 31599 A3 A3 Aegean Airlines 22099 A7 A7 Air Plus Comet 25099 AA AA American Airlines 81028 AAA1 AAA Ansett Air Australia (Dump) 50099 AAA2 AAA Ansett New Zealand (Dump) -

UK National Report (WP 2 - Deliverable 2.2)
UK National report (WP 2 - Deliverable 2.2) Pictures: Inshore fishing boats, Cornwall & Dairy cow, Somerset Authors: Damian MAYE, James KIRWAN, Mauro VIGANI, Dilshaad BUNDHOO and Hannah CHISWELL Organisations April 2018 H2020-SFS-2014-2 SUFISA Grant agreement 635577 1 UK National report Contents EXECUTIVE SUMMARY ...................................................................................................... 12 1 Introduction and methods ........................................................................... 40 2 Media Content Analysis ............................................................................... 42 2.1 Introduction ............................................................................................................. 42 2.2 The predominance of price volatility in media discourses about UK agriculture .... 42 2.3 Inshore fisheries ...................................................................................................... 43 2.4 The dairy sector ....................................................................................................... 46 3 Brexit and the UK agri-food sector ................................................................ 50 3.1 Brexit: introduction ................................................................................................. 50 3.2 Brexit: fisheries, including inshore fisheries ............................................................ 53 3.2.1 Fisheries management ................................................................................... -
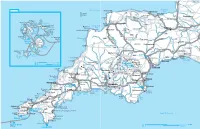
Devon-Cornwall.Pdf
Bridgwater Isles of Scilly Bristol Channel Ilfracombe #\ Minehead Bay /·A39 #\ Lundy Watchet #÷ #\ Island R Dunkery Exmoor Beacon Glastonbury Croyde National 0¸A358 #\ #\ 666Park Bridgwater #\ St Helen Ù# #\ Braunton Shipman's Tean Head St Martin's Saunton #]Barnstaple Bay Sands 0¸A396 Hell Chapel Barnstaple St Martin's Downs Hartland Bay Bay Lawrence's Point Taunton #\ T South#\ #] Tresco Bay Eastern Clovelly Bideford a Bryher Hartland Abbey â# #\ w SOMERSET Crow Isles Molton 0¸B3227 Rushy Bar /·M5 Bay Crow 6660¸A361 A377 Samson Sound 0¸ To Penzance 0¸A388 Chulmleigh 5 Widemouth #\ 0¸A303 # (20mi) Tiverton #\ e ‚ Bay 0¸A386 Isles of St g d Chard Mary's i #\ Scilly Atlantic Ocean r Hugh r DEVON Cullompton #\ #\ o Town Porth Bude #\ T Porth- Hellick #\ Hatherleigh /·M5 DORSET Cressa Honiton #\ Annet St Agnes Gugh Peninnis /·A35 #\ Axminster Head Okehampton #\ Bridport St Mary's /·A30 Exeter #\ Western Sound #^#4 #\ Lyme 0¸A3052 #\ Rocks Boscastle#\ Powderham Regis RHigh Seaton Willhays Chagford #\ Lyme Bay #\ #\ 0¸B3212 Castle Tintagel V# Sidmouth 05km Launceston Range #\ Moreton- 44 e# #\ Lydford Dartmoor â#Ala 0 2.5 miles Brown 666#\ Danger hampstead Ronde National R Willy /·A30 Area #÷3 #] R #\ Park Exmouth Trevose Rough Vixen /·A39 #\ Head Tor â#Jamaica Tor Postbridge Widecombe- 44 Padstow Tavistock R #\ Inn #] #\ in-the-Moor #\ #\ #\ Wadebridge CORNWALL Princetown Newton Abbot Bedruthan Newquay Ashburton Steps #\ Buckland â# Airport Bodmin Liskeard Torquay Watergate #– #\ 666Abbey Lanhydrock â# #] Bay Paignton House Totnes #\ Newquay -

Elasmobranchs & Commercial Fisheries Around the British Isles
Elasmobranchs & Commercial Fisheries around the British Isles: Spatial and Temporal Dynamics JOANA FERNANDES DA SILVA Dissertação de Mestrado em Ciências do Mar 2009 JOANA FERNANDES DA SILVA Elasmobranchs & Commercial Fisheries around the British Isles: Spatial and Temporal dynamics Dissertação de Candidatura ao grau de Mestre em Ciências do Mar – Recursos Marinhos submetida ao Instituto de Ciências Biomédicas de Abel Salazar da Universidade do Porto. Orientador – Doutor Jim Ellis Categoria – Marine Ecologist Afiliação – Centre for Environment, Fisheries & Aquaculture Science (CEFAS) Co-orientadora – Doutora Ivone Figueiredo Categoria – Researcher Afiliação – Instituto das Pescas da Investigação e do Mar (INIAP/IPIMAR) Sumário executivo Os peixes elasmobrânquios desempenham um papel importante nos ecossistemas marinhos e as características dos seus ciclos de vida tornam-nos muito vulnearáveis à exploração comercial. Embora, existam algumas pescarias dirigidas a espécies de elasmobrânquios com carácter sazonal e/ou local ao largo das ilhas Britânicas, os elasmobrânquios são comunemente capturados em pescarias mistas demersais. A pescaria da palangre dirigida a espécies demersais é conhecida por ser altamente selectiva para certas espécies de elasmobrânquios, embora os padrões espaciais e temporais destas pescarias não tenham, ainda, sido descritos. Por conseguinte, uma descrição geral destas pescarias ao largo das ilhas britânicas foi conduzida. As principais zonas, em termos de desembarques na pescaria da palangre demersal (1990-2007) foram o sul do Mar do Norte (ICES IVc), Mar da Irlanda (VIIa) e Mar do Norte central (IVb). Enquanto que, as principais espécies capturadas nestas pescarias são o galhudo- malhado, bacalhau do Atlântico, raias e safio. Desde 2007, as capturas acessórias de raias no Mar do Norte têm uma quota de 25%, a qual foi revista recentemente, sendo apenas obrigatória para as embarcações com mais de 15 m de comprimento total (Regulamento CE n. -

Pendennis Regatta
Cornwall Rowing Association Regatta Calendar – (Final) - 2016 Event Notes Organiser Venue Date March/April Good Friday Race Penryn RC Boscawen Park, Good Friday Truro > Truro 25th March - Gillan to Helford Race - Helford River PGC Gillan > Helford Easter Monday (Ben Collins Sheild) (Ran Dans only) 28th March - Late pm Debbie Rendle Memorial CRA Truro (Boscawen Easter Monday – 28th Park) > Pandora March – 09:00 Start Rock – Ran Dan Race Rock RC Wadebridge>Rock Sunday 17th April (15:00 Hours) May CRA Veterans Skiff CRA Stithians Sunday - 15th May Championships - Regatta Trelawney Regatta CPE Trelawney RC Stithians Sunday - 22nd May St Germans Regatta CPE St Germans SC Cawsand Saturday - 28th May June St Germans “Help the St Germans SC Saturday -18th June Heroes” - Sponsored Row Durgan Regatta CPE CRA Durgan Sunday - 26th June Cornwall Schools Games Cornwall Sports Mylor Wed - 29th June Partnership July Mousehole Regatta CPE Mousehole RC Mousehole Sunday 3rd July Point & Penpol Regatta Village Devoran PGC Point Mon 4th July - Evening Saltash Regatta CPE Saltash RC Saltash Sun 10th July Penryn Regatta CPE Penryn RC Turnaware Bar Sat 16th July Penryn Regatta - (Reserve) CPE Penryn RC Turnaware Bar Sat 30th July August Polperro Regatta CPE Polperro RC Polperro Sunday 7th August George Angove CPEC CPE CRA Siblyback Lake Sunday 14th August Memorial Regatta Calstock Regatta CPE Calstock RC Calstock Sat/Sun – 27th/28th August Cadgwith Village Regatta Village Cadgwith PGC Cadgwith Monday 29th August (Incl. Skiffs & Working Boats) September Polperro Regatta (Reserve) CPE Polperro RC Polperro Sun 4thSeptember CPE = Championship Point Events Reserve Date = Green 25/02/16 . -

Book of Abstracts: 6Th Annual Meeting of the European Elasmobranch Association 2002
6th Annual European Elasmobranch Association Conference Book of Abstracts: 6th Annual Meeting of the European Elasmobranch Association 2002 9.35 –10.45 Welsh Skate and Ray Project H. Jones, N.K. Dulvy, P.J. Coates and C. Eno Annual landings of ray species (Rajidae) in England and Wales have fallen from around 18,000 to 3,000 tonnes over the last 40 years. The cause of the decline is believed to be largely due to over-fishing, particularly from the use of trawl gears. The Bristol Channel area is of particular importance and used to account for around 25-30% of UK landings. Common skate (Dipturus batis) was once a frequent catch but is now commercially extinct. Although data is incomplete larger ray species Raja brachyura (blonde) and R. clavata (thornback) have apparently declined in both abundance and average size, to be partly replaced by the smaller species R. montagui (spotted) and Leucoraja naevus (Cuckoo). Fishery Managers face the challenge of identifying practical conservation measures that need to be acceptable to the majority if they are to be effective. Options include: • Size restrictions i.e. minimum and/or maximum size (and options by species or sex and whether applied to part or whole fish). • Restricted licencing of vessel effort or catch (quotas). • Technical measures e.g. mesh sizes, vessel size, gear type. • Gear restrictions (e.g. by location and/or time). • No take or limited take zones (e.g. no taking of female rays). As well as being of commercial value, rays are also of wider recreational and conservation interest and a unique group of interested parties in Wales have come together to address a "common cause". -

Grade Ruan Parish Plan
Grade Ruan Parish Plan enhancing our lives, now and for the future Index Title Page Associated Title Page Associated No tables No tables Objectives: 1 Cultural Activities and the Arts 14 The Purpose of the Strategy 1 Cultural Activities and the Arts Table 15 D The Life of this Strategy 1 Leisure 16 The Parish of Grade Ruan 2 Leisure Table 17 E Present Amenities 3 Health 18 History Notes 4 Health Table 18 F The Story so far 5 Housing 19 Environment 8 Housing Table 19 G Environment Table 9 A Employment & Training 20 Traffic 10 Employment & Training Table 21 H Traffic Table 11 B Summary 22 Community Facilities 12 List of other Potential Projects 23, 24 Community Facilities Table 13 C Glossary & Acknowledgements 25 Photo Index Photo Page No Photo Page No The Parish of Grade Ruan 2 Grade Ruan Under Fives Group 12 Grade Ruan C of E Primary School 3 Rozen Furniture 13 St Ruan Church 3 Cadgwith Thespians Midsummer Night’s Dream 14 The old pavilion building 4 Costume/set preparations for the production 14 Fishing Boats at Cadgwith 4 Another scene from the 2002 production 15 Goonhilly Satellite dishes 5 New multi-purpose court at Recreation ground 16 St. Ruan’s Well 5 Ruan Minor Play Area at Recreation ground 16 Have Your Say Day 6 Gig rowers off Cadgwith 16 Grade Ruan Village Hall 6 Gig Clubhouse prior to refurbishment 17 GRAB Logo 7 Ruan Minor Doctors surgery 18 Entrance to the Village of Ruan Minor 8 Doctors plaque at surgery 18 Ruan Minor post office and Spar shop 8 New build housing at Long Moor (1) 19 Tree planting at Cadgwith car park 9