Manabe and Wetherald (1967)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Warming Climate Records
ATM S 111: Global Warming Climate Records Jennifer Fletcher Day 24: July 26 2010 Reading For today: “Keeping Track” (Climate Records) pp. 171-192 For tomorrow/Wednesday: “The Long View” (Paleoclimate) pp. 193-226. The Instrumental Record from NASA Global temperature since 1880 Ten warmest years: 2001-2009 and 1998 (biggest El Niño ever) Separation into Northern/ Southern Hemispheres N. Hem. has warmed more (1o C vs 0.8o C globally) S. Hem. has warmed more steadily though Cooling in the record from 1940-1975 essentially only in the N. Hem. record (this is likely due to aerosol cooling) Temperature estimates from other groups CRUTEM3 (RG calls this UEA) NCDC (RG calls this NOAA) GISS (RG calls this NASA) yet another group Surface air temperature over land Thermometer between 1.25-2 m (4-6.5 ft) above ground White colored to reflect away direct sunlight Slats to ensure fresh air circulation “Stevenson screen”: invented by Robert Louis Stevenson’s dad Thomas Yearly average Central England temperature record (since 1659) Temperatures over Land Only NASA separates their analysis into land station data only (not including ship measurements) Warming in the station data record is larger than in the full record (1.1o C as opposed to 0.8o C) Sea surface temperature measurements Standard bucket Canvas bucket Insulated bucket (~1891) (pre WWII) (now) “Bucket” temperature: older style subject to evaporative cooling Starting around WWII: many temperature measurements taken from condenser intake pipe instead of from buckets. Typically 0.5 C warmer -

Atmospheric Thermal and Dynamic Vertical Structures of Summer Hourly Precipitation in Jiulong of the Tibetan Plateau
atmosphere Article Atmospheric Thermal and Dynamic Vertical Structures of Summer Hourly Precipitation in Jiulong of the Tibetan Plateau Yonglan Tang 1, Guirong Xu 1,*, Rong Wan 1, Xiaofang Wang 1, Junchao Wang 1 and Ping Li 2 1 Hubei Key Laboratory for Heavy Rain Monitoring and Warning Research, Institute of Heavy Rain, China Meteorological Administration, Wuhan 430205, China; [email protected] (Y.T.); [email protected] (R.W.); [email protected] (X.W.); [email protected] (J.W.) 2 Heavy Rain and Drought-Flood Disasters in Plateau and Basin Key Laboratory of Sichuan Province, Institute of Plateau Meteorology, China Meteorological Administration, Chengdu 610072, China; [email protected] * Correspondence: [email protected]; Tel.: +86-27-8180-4913 Abstract: It is an important to study atmospheric thermal and dynamic vertical structures over the Tibetan Plateau (TP) and their impact on precipitation by using long-term observation at represen- tative stations. This study exhibits the observational facts of summer precipitation variation on subdiurnal scale and its atmospheric thermal and dynamic vertical structures over the TP with hourly precipitation and intensive soundings in Jiulong during 2013–2020. It is found that precipitation amount and frequency are low in the daytime and high in the nighttime, and hourly precipitation greater than 1 mm mostly occurs at nighttime. Weak precipitation during the daytime may be caused by air advection, and strong precipitation at nighttime may be closely related with air convection. Both humidity and wind speed profiles show obvious fluctuation when precipitation occurs, and the greater the precipitation intensity, the larger the fluctuation. Moreover, the fluctuation of wind speed Citation: Tang, Y.; Xu, G.; Wan, R.; Wang, X.; Wang, J.; Li, P. -

The Measurement of Tropospheric
EPJ Web of Conferences 119,27004 (2016) DOI: 10.1051/epjconf/201611927004 ILRC 27 THE MEASUREMENT OF TROPOSPHERIC TEMPERATURE PROFILES USING RAYLEIGH-BRILLOUIN SCATTERING: RESULTS FROM LABORATORY AND ATMOSPHERIC STUDIES Benjamin Witschas1*, Oliver Reitebuch1, Christian Lemmerz1, Pau Gomez Kableka1, Sergey Kondratyev2, Ziyu Gu3, Wim Ubachs3 1German Aerospace Center (DLR), Institute of Atmospheric Physics, 82234 Oberpfaffenhofen, Germany 2Angstrom Ltd., 630090 Inzhenernaya16, Novosibirsk, Russia 3Department of Physics and Astronomy, LaserLaB, VU University, 1081 HV Amsterdam, Netherlands *Email: [email protected] ABSTRACT are only applicable to stratospheric or meso- In this letter, we suggest a new method for spheric measurements because they can only be measuring tropospheric temperature profiles using applied in air masses that do not contain aerosols, Rayleigh-Brillouin (RB) scattering. We report on or where appropriate fluorescing metal atoms laboratory RB scattering measurements in air, exist. In contrast, Raman lidars can be applied for demonstrating that temperature can be retrieved tropospheric measurements [2]. Nevertheless, it from RB spectra with an absolute accuracy of has to be mentioned that the Raman scattering better than 2 K. In addition, we show temperature cross section is quite low. Thus, powerful lasers, profiles from 2 km to 15.3 km derived from RB sophisticated background filters, or night-time spectra, measured with a high spectral resolution operation are required to obtain reliable results. In lidar during daytime. A comparison with particular, the rotational Raman differential radiosonde temperature measurements shows backscattering cross section (considering Stokes reasonable agreement. In cloud-free conditions, and anti-Stokes branches) is about a factor of 50 the temperature difference reaches up to 5 K smaller than the one of Rayleigh scattering [3]. -

A Simple Lumped Model to Convert Air Temperature Into Surface Water
EGU Journal Logos (RGB) Open Access Open Access Open Access Advances in Annales Nonlinear Processes Geosciences Geophysicae in Geophysics Open Access Open Access Natural Hazards Natural Hazards and Earth System and Earth System Sciences Sciences Discussions Open Access Open Access Atmospheric Atmospheric Chemistry Chemistry and Physics and Physics Discussions Open Access Open Access Atmospheric Atmospheric Measurement Measurement Techniques Techniques Discussions Open Access Open Access Biogeosciences Biogeosciences Discussions Open Access Open Access Climate Climate of the Past of the Past Discussions Open Access Open Access Earth System Earth System Dynamics Dynamics Discussions Open Access Geoscientific Geoscientific Open Access Instrumentation Instrumentation Methods and Methods and Data Systems Data Systems Discussions Open Access Open Access Geoscientific Geoscientific Model Development Model Development Discussions Open Access Open Access Hydrol. Earth Syst. Sci., 17, 3323–3338, 2013 Hydrology and Hydrology and www.hydrol-earth-syst-sci.net/17/3323/2013/ doi:10.5194/hess-17-3323-2013 Earth System Earth System © Author(s) 2013. CC Attribution 3.0 License. Sciences Sciences Discussions Open Access Open Access Ocean Science Ocean Science Discussions A simple lumped model to convert air temperature into surface Open Access Open Access water temperature in lakes Solid Earth Solid Earth Discussions S. Piccolroaz, M. Toffolon, and B. Majone Department of Civil, Environmental and Mechanical Engineering, University of Trento, Italy Open Access Open Access Correspondence to: S. Piccolroaz ([email protected]) The Cryosphere Received: 23 January 2013 – Published in Hydrol. Earth Syst. Sci. Discuss.: 5 March 2013The Cryosphere Discussions Revised: 4 July 2013 – Accepted: 20 July 2013 – Published: 27 August 2013 Abstract. Water temperature in lakes is governed by a com- show remarkable agreement with measurements over the en- plex heat budget, where the estimation of the single fluxes tire data period. -

Synoptic Atmospheric Conditions, Land Cover, and Equivalent
Western Kentucky University TopSCHOLAR® Masters Theses & Specialist Projects Graduate School Spring 2017 Synoptic Atmospheric Conditions, Land Cover, and Equivalent Temperature Variations in Kentucky Dorothy Yemaa Na-Yemeh Western Kentucky University, [email protected] Follow this and additional works at: http://digitalcommons.wku.edu/theses Part of the Climate Commons, Geology Commons, and the Geophysics and Seismology Commons Recommended Citation Na-Yemeh, Dorothy Yemaa, "Synoptic Atmospheric Conditions, Land Cover, and Equivalent Temperature Variations in Kentucky" (2017). Masters Theses & Specialist Projects. Paper 1930. http://digitalcommons.wku.edu/theses/1930 This Thesis is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in Masters Theses & Specialist Projects by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. SYNOPTIC ATMOSPHERIC CONDITIONS, LAND COVER, AND EQUIVALENT TEMPERATURE VARIATIONS IN KENTUCKY A Thesis Presented to The Graduate Faculty of the Department of Geology and Geography Western Kentucky University Bowling Green, Kentucky In Partial Fulfillment of the Requirements for the Degree Master of Science By Dolly Na-Yemeh May 2017 SYNOPTIC ATMOSPHERIC CONDITIONS, LAND COVER AND EQUIVALENT TEMPERATURE VARIATIONS IN KENTUCKY ACKNOWLEDGMENTS Psalm 9:1 I will give thanks to you, LORD, with all my heart; I will tell of your wonderful deeds. I had the support of many people in the process of earning the M.S. degree in the Department of Geography and Geology at Western Kentucky University. First, I would like to thank my thesis advisor, Dr. Rezaul Mahmood, for his guidance, support, and encouragement throughout this program. The door to Dr. -

Dry Adiabatic Temperature Lapse Rate
ATMO 551a Fall 2010 Dry Adiabatic Temperature Lapse Rate As we discussed earlier in this class, a key feature of thick atmospheres (where thick means atmospheres with pressures greater than 100-200 mb) is temperature decreases with increasing altitude at higher pressures defining the troposphere of these planets. We want to understand why tropospheric temperatures systematically decrease with altitude and what the rate of decrease is. The first order explanation is the dry adiabatic lapse rate. An adiabatic process means no heat is exchanged in the process. For this to be the case, the process must be “fast” so that no heat is exchanged with the environment. So in the first law of thermodynamics, we can anticipate that we will set the dQ term equal to zero. To get at the rate at which temperature decreases with altitude in the troposphere, we need to introduce some atmospheric relation that defines a dependence on altitude. This relation is the hydrostatic relation we have discussed previously. In summary, the adiabatic lapse rate will emerge from combining the hydrostatic relation and the first law of thermodynamics with the heat transfer term, dQ, set to zero. The gravity side The hydrostatic relation is dP = −g ρ dz (1) which relates pressure to altitude. We rearrange this as dP −g dz = = α dP (2) € ρ where α = 1/ρ is known as the specific volume which is the volume per unit mass. This is the equation we will use in a moment in deriving the adiabatic lapse rate. Notice that € F dW dE g dz ≡ dΦ = g dz = g = g (3) m m m where Φ is known as the geopotential, Fg is the force of gravity and dWg is the work done by gravity. -

Temperature and Relative Humidity Profile Retrieval from Fengyun-3D
remote sensing Article Temperature and Relative Humidity Profile Retrieval from Fengyun-3D/HIRAS in the Arctic Region Jingjing Hu 1,2, Yansong Bao 1,2,*, Jian Liu 3, Hui Liu 3 , George P. Petropoulos 4 , Petros Katsafados 4 , Liuhua Zhu 1,2 and Xi Cai 1,2 1 Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters, CMA Key Laboratory for Aerosol-Cloud-Precipitation, Nanjing University of Information Science & Technology, Nanjing 210044, China; [email protected] (J.H.); [email protected] (L.Z.); [email protected] (X.C.) 2 School of Atmospheric Physics, Nanjing University of Information Science & Technology, Nanjing 210044, China 3 National Satellite Meteorological Center, Key Laboratory of Radiometric Calibration and Validation for Environmental Satellites, China Meteorological Administration, Beijing 100081, China; [email protected] (J.L.); [email protected] (H.L.) 4 Department of Geography, Harokopio University of Athens, EI. Venizelou 70, Kallithea, 17671 Athens, Greece; [email protected] (G.P.P.); [email protected] (P.K.) * Correspondence: [email protected] Abstract: The acquisition of real-time temperature and relative humidity (RH) profiles in the Arctic is of great significance for the study of the Arctic’s climate and Arctic scientific research. However, the operational algorithm of Fengyun-3D only takes into account areas within 60◦N, the innovation of this work is that a new technique based on Neural Network (NN) algorithm was proposed, which can retrieve these parameters in real time from the Fengyun-3D Hyperspectral Infrared Radiation Citation: Hu, J.; Bao, Y.; Liu, J.; Liu, Atmospheric Sounding (HIRAS) observations in the Arctic region. -

Vertical Stability of the Atmosphere
AVIATION REFERENCE MATERIAL Vertical Stability of the Atmosphere Bureau of Meteorology › Aviation Meteorological Services Vertical motion in the atmosphere is The DALR is the rate at which the largely responsible for turbulence and temperature of a dry (unsaturated) cloud formation. air parcel changes as it ascends or descends through the atmosphere.It The strength of vertical motion is is approximately 3°C per 1000 feet. mostly determined by the vertical stability of the atmosphere. A stable The SALR is the rate at which the atmosphere will tend to resist vertical temperature of a moist (saturated) motion, while an unstable atmosphere air parcel changes as it ascends or will assist it. When the atmosphere descends through the atmosphere. neither resists nor assists vertical The SALR is often taken as 1.5°C per motion it is said to have neutral 1000 feet, although the actual figure stability. varies according to the amount of water vapour present and also the temperature of the air parcel (an air Adiabatic Processes and parcel at a higher temperature can Lapse Rates contain more water vapour than when To explain the concept of stability in at a lower temperature). the atmosphere, it is useful to consider Vertical motion of air is what happens to an imaginary The SALR is less than the DALR an important driver of parcel of air displaced vertically from because as a parcel of saturated air weather. Sometimes rising one level to another. During such ascends and cools, water vapour air is made visible by the displacement it is assumed that condenses into water droplets, development of clouds or the parcel undergoes an adiabatic releasing latent heat into the parcel, which slows the cooling (and helps to by the rising dust in dust process, i.e. -

Atmosphere Aloft
Project ATMOSPHERE This guide is one of a series produced by Project ATMOSPHERE, an initiative of the American Meteorological Society. Project ATMOSPHERE has created and trained a network of resource agents who provide nationwide leadership in precollege atmospheric environment education. To support these agents in their teacher training, Project ATMOSPHERE develops and produces teacher’s guides and other educational materials. For further information, and additional background on the American Meteorological Society’s Education Program, please contact: American Meteorological Society Education Program 1200 New York Ave., NW, Ste. 500 Washington, DC 20005-3928 www.ametsoc.org/amsedu This material is based upon work initially supported by the National Science Foundation under Grant No. TPE-9340055. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the National Science Foundation. © 2012 American Meteorological Society (Permission is hereby granted for the reproduction of materials contained in this publication for non-commercial use in schools on the condition their source is acknowledged.) 2 Foreword This guide has been prepared to introduce fundamental understandings about the guide topic. This guide is organized as follows: Introduction This is a narrative summary of background information to introduce the topic. Basic Understandings Basic understandings are statements of principles, concepts, and information. The basic understandings represent material to be mastered by the learner, and can be especially helpful in devising learning activities in writing learning objectives and test items. They are numbered so they can be keyed with activities, objectives and test items. Activities These are related investigations. -

A Glossary for Biometeorology
Int J Biometeorol DOI 10.1007/s00484-013-0729-9 ICB 2011 - STUDENTS / NEW PROFESSIONALS A glossary for biometeorology Simon N. Gosling & Erin K. Bryce & P. Grady Dixon & Katharina M. A. Gabriel & Elaine Y.Gosling & Jonathan M. Hanes & David M. Hondula & Liang Liang & Priscilla Ayleen Bustos Mac Lean & Stefan Muthers & Sheila Tavares Nascimento & Martina Petralli & Jennifer K. Vanos & Eva R. Wanka Received: 30 October 2012 /Revised: 22 August 2013 /Accepted: 26 August 2013 # The Author(s) 2013. This article is published with open access at Springerlink.com Abstract Here we present, for the first time, a glossary of berevisitedincomingyears,updatingtermsandaddingnew biometeorological terms. The glossary aims to address the need terms, as appropriate. The glossary is intended to provide a for a reliable source of biometeorological definitions, thereby useful resource to the biometeorology community, and to this facilitating communication and mutual understanding in this end, readers are encouraged to contact the lead author to suggest rapidly expanding field. A total of 171 terms are defined, with additional terms for inclusion in later versions of the glossary as reference to 234 citations. It is anticipated that the glossary will a result of new and emerging developments in the field. S. N. Gosling (*) L. Liang School of Geography, University of Nottingham, Nottingham NG7 Department of Geography, University of Kentucky, Lexington, 2RD, UK KY, USA e-mail: [email protected] E. K. Bryce P. A. Bustos Mac Lean Department of Anthropology, University of Toronto, Department of Animal Science, Universidade Estadual de Maringá Toronto, ON, Canada (UEM), Maringa, Paraná, Brazil P. G. Dixon S. -
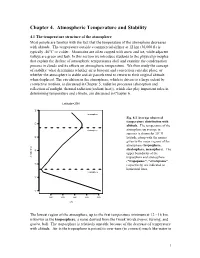
Chapter 4. Atmospheric Temperature and Stability
Chapter 4. Atmospheric Temperature and Stability 4.1 The temperature structure of the atmosphere Most people are familiar with the fact that the temperature of the atmosphere decreases with altitude. The temperature outside a commercial airliner at 12 km (36,000 ft) is typically -40°C or colder. Mountains are often capped with snow and ice, while adjacent valleys are green and lush. In this section we introduce students to the physical principles that explain the decline of atmospheric temperatures aloft and examine the condensation process in clouds and its effects on atmospheric temperature. We then study the concept of stability: what determines whether air is buoyant and convection can take place, or whether the atmosphere is stable and air parcels tend to return to their original altitude when displaced. The circulation in the atmosphere, which is driven to a large extent by convective motions, is discussed in Chapter 5; radiative processes (absorption and reflection of sunlight, thermal radiation (radiant heat)), which also play important roles in determining temperature and climate, are discussed in Chapter 6. Latitude=30N mesosphere Fig. 4.1 Average observed temperature distribution with altitude. The temperature of the stratosphere atmosphere on average in summer is shown for 30° N latitude, along with the names given to the major regions of the atmosphere (troposphere, stratosphere, mesosphere). The upper boundaries of the Altitude (km) troposphere and stratosphere ("tropopause", "stratopause", respectively) are indicated as horizontal lines. troposphere 0 102030405060 220 240 260 280 300 T (K) The lowest region of the atmosphere, up to the first temperature minimum at 12 - 16 km, is known as the troposphere, a name derived from the Greek words tropos, turning, and spaira, ball. -

Correlation Between Atmospheric Temperature and Soil Temperature: a Case Study for Dhaka, Bangladesh
Atmospheric and Climate Sciences, 2015, 5, 200-208 Published Online July 2015 in SciRes. http://www.scirp.org/journal/acs http://dx.doi.org/10.4236/acs.2015.53014 Correlation between Atmospheric Temperature and Soil Temperature: A Case Study for Dhaka, Bangladesh Khandaker Iftekharul Islam, Anisuzzaman Khan, Tanaz Islam Department of Civil Engineering, IUBAT─International University of Business Agriculture and Technology, Dhaka, Bangladesh Email: [email protected] Received 15 April 2015; accepted 2 June 2015; published 5 June 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract The main intention of this study was to substantiate the relation between atmospheric tempera- ture and soil temperature within a system boundary. The study focused on coefficient of correla- tion that demonstrates the association between two variables. The earth surface temperature is anticipated to be affected by a set of meteorological parameters. Atmospheric temperature, hu- midity, precipitation and solar radiation directly influence the adjacent soil and the extent of im- pact must be varied at different part of the earth because of multiple factors. The primary step to validate the correlation was to collect two series of data for two variables i.e. atmospheric tem- perature and soil temperature of last ten years from 2003 to 2012. The coefficient of correlation was determined through Pearson’s distribution whereas atmospheric temperature was consi- dered as independent and soil temperature was dependent variable. The coefficients were calcu- lated distinctly taking the soil temperature not only at several depths but also at separate faces of a day commensurate to overlying air temperature.