Experimental Design 1 Running Head
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Internal Validity Is About Causal Interpretability
Internal Validity is about Causal Interpretability Before we can discuss Internal Validity, we have to discuss different types of Internal Validity variables and review causal RH:s and the evidence needed to support them… Every behavior/measure used in a research study is either a ... Constant -- all the participants in the study have the same value on that behavior/measure or a ... • Measured & Manipulated Variables & Constants Variable -- when at least some of the participants in the study • Causes, Effects, Controls & Confounds have different values on that behavior/measure • Components of Internal Validity • “Creating” initial equivalence and every behavior/measure is either … • “Maintaining” ongoing equivalence Measured -- the value of that behavior/measure is obtained by • Interrelationships between Internal Validity & External Validity observation or self-report of the participant (often called “subject constant/variable”) or it is … Manipulated -- the value of that behavior/measure is controlled, delivered, determined, etc., by the researcher (often called “procedural constant/variable”) So, every behavior/measure in any study is one of four types…. constant variable measured measured (subject) measured (subject) constant variable manipulated manipulated manipulated (procedural) constant (procedural) variable Identify each of the following (as one of the four above, duh!)… • Participants reported practicing between 3 and 10 times • All participants were given the same set of words to memorize • Each participant reported they were a Psyc major • Each participant was given either the “homicide” or the “self- defense” vignette to read From before... Circle the manipulated/causal & underline measured/effect variable in each • Causal RH: -- differences in the amount or kind of one behavior cause/produce/create/change/etc. -

Validity and Reliability of the Questionnaire for Compliance with Standard Precaution for Nurses
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cadernos Espinosanos (E-Journal) Rev Saúde Pública 2015;49:87 Original Articles DOI:10.1590/S0034-8910.2015049005975 Marília Duarte ValimI Validity and reliability of the Maria Helena Palucci MarzialeII Miyeko HayashidaII Questionnaire for Compliance Fernanda Ludmilla Rossi RochaIII with Standard Precaution Jair Lício Ferreira SantosIV ABSTRACT OBJECTIVE: To evaluate the validity and reliability of the Questionnaire for Compliance with Standard Precaution for nurses. METHODS: This methodological study was conducted with 121 nurses from health care facilities in Sao Paulo’s countryside, who were represented by two high-complexity and by three average-complexity health care facilities. Internal consistency was calculated using Cronbach’s alpha and stability was calculated by the intraclass correlation coefficient, through test-retest. Convergent, discriminant, and known-groups construct validity techniques were conducted. RESULTS: The questionnaire was found to be reliable (Cronbach’s alpha: 0.80; intraclass correlation coefficient: (0.97) In regards to the convergent and discriminant construct validity, strong correlation was found between compliance to standard precautions, the perception of a safe environment, and the smaller perception of obstacles to follow such precautions (r = 0.614 and r = 0.537, respectively). The nurses who were trained on the standard precautions and worked on the health care facilities of higher complexity were shown to comply more (p = 0.028 and p = 0.006, respectively). CONCLUSIONS: The Brazilian version of the Questionnaire for I Departamento de Enfermagem. Centro Compliance with Standard Precaution was shown to be valid and reliable. -

Comorbidity Scores
Bias Introduction of issue and background papers Sebastian Schneeweiss, MD, ScD Professor of Medicine and Epidemiology Division of Pharmacoepidemiology and Pharmacoeconomics, Dept of Medicine, Brigham & Women’s Hospital/ Harvard Medical School 1 Potential conflicts of interest PI, Brigham & Women’s Hospital DEcIDE Center for Comparative Effectiveness Research (AHRQ) PI, DEcIDE Methods Center (AHRQ) Co-Chair, Methods Core of the Mini Sentinel System (FDA) Member, national PCORI Methods Committee No paid consulting or speaker fees from pharmaceutical manufacturers Consulting in past year: . WHISCON LLC, Booz&Co, Aetion Investigator-initiated research grants to the Brigham from Pfizer, Novartis, Boehringer-Ingelheim Multiple grants from NIH 2 Objective of Comparative Effectiveness Research Efficacy Effectiveness* (Can it work?) (Does it work in routine care?) Placebo Most RCTs comparison for drug (or usual care) approval Active Goal of comparison (head-to-head) CER Effectiveness = Efficacy X Adherence X Subgroup effects (+/-) RCT Reality of routine care 3 * Cochrane A. Nuffield Provincial Trust, 1972 CER Baseline Non- randomization randomized Primary Secondary Primary Secondary data data data data 4 Challenges of observational research Measurement /surveillance-related biases . Informative missingness/ misclassification Selection-related biases . Confounding . Informative treatment changes/discontinuations Time-related biases . Immortal time bias . Temporality . Effect window (Multiple comparisons) 5 Informative missingness: -
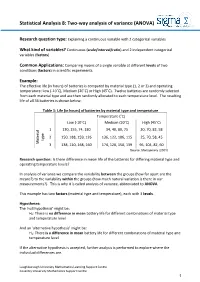
Statistical Analysis 8: Two-Way Analysis of Variance (ANOVA)
Statistical Analysis 8: Two-way analysis of variance (ANOVA) Research question type: Explaining a continuous variable with 2 categorical variables What kind of variables? Continuous (scale/interval/ratio) and 2 independent categorical variables (factors) Common Applications: Comparing means of a single variable at different levels of two conditions (factors) in scientific experiments. Example: The effective life (in hours) of batteries is compared by material type (1, 2 or 3) and operating temperature: Low (-10˚C), Medium (20˚C) or High (45˚C). Twelve batteries are randomly selected from each material type and are then randomly allocated to each temperature level. The resulting life of all 36 batteries is shown below: Table 1: Life (in hours) of batteries by material type and temperature Temperature (˚C) Low (-10˚C) Medium (20˚C) High (45˚C) 1 130, 155, 74, 180 34, 40, 80, 75 20, 70, 82, 58 2 150, 188, 159, 126 136, 122, 106, 115 25, 70, 58, 45 type Material 3 138, 110, 168, 160 174, 120, 150, 139 96, 104, 82, 60 Source: Montgomery (2001) Research question: Is there difference in mean life of the batteries for differing material type and operating temperature levels? In analysis of variance we compare the variability between the groups (how far apart are the means?) to the variability within the groups (how much natural variation is there in our measurements?). This is why it is called analysis of variance, abbreviated to ANOVA. This example has two factors (material type and temperature), each with 3 levels. Hypotheses: The 'null hypothesis' might be: H0: There is no difference in mean battery life for different combinations of material type and temperature level And an 'alternative hypothesis' might be: H1: There is a difference in mean battery life for different combinations of material type and temperature level If the alternative hypothesis is accepted, further analysis is performed to explore where the individual differences are. -

Survey Experiments
IU Workshop in Methods – 2019 Survey Experiments Testing Causality in Diverse Samples Trenton D. Mize Department of Sociology & Advanced Methodologies (AMAP) Purdue University Survey Experiments Page 1 Survey Experiments Page 2 Contents INTRODUCTION ............................................................................................................................................................................ 8 Overview .............................................................................................................................................................................. 8 What is a survey experiment? .................................................................................................................................... 9 What is an experiment?.............................................................................................................................................. 10 Independent and dependent variables ................................................................................................................. 11 Experimental Conditions ............................................................................................................................................. 12 WHY CONDUCT A SURVEY EXPERIMENT? ........................................................................................................................... 13 Internal, external, and construct validity .......................................................................................................... -

Analysis of Variance and Analysis of Variance and Design of Experiments of Experiments-I
Analysis of Variance and Design of Experimentseriments--II MODULE ––IVIV LECTURE - 19 EXPERIMENTAL DESIGNS AND THEIR ANALYSIS Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur 2 Design of experiment means how to design an experiment in the sense that how the observations or measurements should be obtained to answer a qqyuery inavalid, efficient and economical way. The desigggning of experiment and the analysis of obtained data are inseparable. If the experiment is designed properly keeping in mind the question, then the data generated is valid and proper analysis of data provides the valid statistical inferences. If the experiment is not well designed, the validity of the statistical inferences is questionable and may be invalid. It is important to understand first the basic terminologies used in the experimental design. Experimental unit For conducting an experiment, the experimental material is divided into smaller parts and each part is referred to as experimental unit. The experimental unit is randomly assigned to a treatment. The phrase “randomly assigned” is very important in this definition. Experiment A way of getting an answer to a question which the experimenter wants to know. Treatment Different objects or procedures which are to be compared in an experiment are called treatments. Sampling unit The object that is measured in an experiment is called the sampling unit. This may be different from the experimental unit. 3 Factor A factor is a variable defining a categorization. A factor can be fixed or random in nature. • A factor is termed as fixed factor if all the levels of interest are included in the experiment. -

The Politics of Random Assignment: Implementing Studies and Impacting Policy
The Politics of Random Assignment: Implementing Studies and Impacting Policy Judith M. Gueron Manpower Demonstration Research Corporation (MDRC) As the only nonacademic presenting a paper at this conference, I see it as my charge to focus on the challenge of implementing random assignment in the field. I will not spend time arguing for the methodological strengths of social experiments or advocating for more such field trials. Others have done so eloquently.1 But I will make my biases clear. For 25 years, I and many of my MDRC colleagues have fought to implement random assignment in diverse arenas and to show that this approach is feasible, ethical, uniquely convincing, and superior for answering certain questions. Our organization is widely credited with being one of the pioneers of this approach, and through its use producing results that are trusted across the political spectrum and that have made a powerful difference in social policy and research practice. So, I am a believer, but not, I hope, a blind one. I do not think that random assignment is a panacea or that it can address all the critical policy questions, or substitute for other types of analysis, or is always appropriate. But I do believe that it offers unique power in answering the “Does it make a difference?” question. With random assignment, you can know something with much greater certainty and, as a result, can more confidently separate fact from advocacy. This paper focuses on implementing experiments. In laying out the ingredients of success, I argue that creative and flexible research design skills are essential, but that just as important are operational and political skills, applied both to marketing the experiment in the first place and to helping interpret and promote its findings down the line. -

Validity and Reliability in Quantitative Studies Evid Based Nurs: First Published As 10.1136/Eb-2015-102129 on 15 May 2015
Research made simple Validity and reliability in quantitative studies Evid Based Nurs: first published as 10.1136/eb-2015-102129 on 15 May 2015. Downloaded from Roberta Heale,1 Alison Twycross2 10.1136/eb-2015-102129 Evidence-based practice includes, in part, implementa- have a high degree of anxiety? In another example, a tion of the findings of well-conducted quality research test of knowledge of medications that requires dosage studies. So being able to critique quantitative research is calculations may instead be testing maths knowledge. 1 School of Nursing, Laurentian an important skill for nurses. Consideration must be There are three types of evidence that can be used to University, Sudbury, Ontario, given not only to the results of the study but also the demonstrate a research instrument has construct Canada 2Faculty of Health and Social rigour of the research. Rigour refers to the extent to validity: which the researchers worked to enhance the quality of Care, London South Bank 1 Homogeneity—meaning that the instrument mea- the studies. In quantitative research, this is achieved University, London, UK sures one construct. through measurement of the validity and reliability.1 2 Convergence—this occurs when the instrument mea- Correspondence to: Validity sures concepts similar to that of other instruments. Dr Roberta Heale, Validity is defined as the extent to which a concept is Although if there are no similar instruments avail- School of Nursing, Laurentian accurately measured in a quantitative study. For able this will not be possible to do. University, Ramsey Lake Road, example, a survey designed to explore depression but Sudbury, Ontario, Canada 3 Theory evidence—this is evident when behaviour is which actually measures anxiety would not be consid- P3E2C6; similar to theoretical propositions of the construct ered valid. -

Experimental Design
UNIVERSITY OF BERGEN Experimental design Sebastian Jentschke UNIVERSITY OF BERGEN Agenda • experiments and causal inference • validity • internal validity • statistical conclusion validity • construct validity • external validity • tradeoffs and priorities PAGE 2 Experiments and causal inference UNIVERSITY OF BERGEN Some definitions a hypothesis often concerns a cause- effect-relationship PAGE 4 UNIVERSITY OF BERGEN Scientific revolution • discovery of America → french revolution: renaissance and enlightenment – Copernicus, Galilei, Newton • empiricism: use observation to correct errors in theory • scientific experimentation: taking a deliberate action [manipulation, vary something] followed by systematic observation of what occured afterwards [effect] controlling extraneous influences that might limit or bias observation: random assignment, control groups • mathematization, institutionalization PAGE 5 UNIVERSITY OF BERGEN Causal relationships Definitions and some philosophy: • causal relationships are recognized intuitively by most people in their daily lives • Locke: „A cause is which makes any other thing, either simple idea, substance or mode, begin to be; and an effect is that, which had its beginning from some other thing“ (1975, p. 325) • Stuart Mill: A causal relationship exists if (a) the cause preceded the effect, (b) the cause was related to the effect, (c) we can find no plausible alternative explanantion for the effect other than the cause. Experiments: (a) manipulate the presumed cause, (b) assess whether variation in the -

Understanding Replication of Experiments in Software Engineering: a Classification Omar S
Understanding replication of experiments in software engineering: A classification Omar S. Gómez a, , Natalia Juristo b,c, Sira Vegas b a Facultad de Matemáticas, Universidad Autónoma de Yucatán, 97119 Mérida, Yucatán, Mexico Facultad de Informática, Universidad Politécnica de Madrid, 28660 Boadilla del Monte, Madrid, Spain c Department of Information Processing Science, University of Oulu, Oulu, Finland abstract Context: Replication plays an important role in experimental disciplines. There are still many uncertain-ties about how to proceed with replications of SE experiments. Should replicators reuse the baseline experiment materials? How much liaison should there be among the original and replicating experiment-ers, if any? What elements of the experimental configuration can be changed for the experiment to be considered a replication rather than a new experiment? Objective: To improve our understanding of SE experiment replication, in this work we propose a classi-fication which is intend to provide experimenters with guidance about what types of replication they can perform. Method: The research approach followed is structured according to the following activities: (1) a litera-ture review of experiment replication in SE and in other disciplines, (2) identification of typical elements that compose an experimental configuration, (3) identification of different replications purposes and (4) development of a classification of experiment replications for SE. Results: We propose a classification of replications which provides experimenters in SE with guidance about what changes can they make in a replication and, based on these, what verification purposes such a replication can serve. The proposed classification helped to accommodate opposing views within a broader framework, it is capable of accounting for less similar replications to more similar ones regarding the baseline experiment. -

Introduction to Difference in Differences (DID) Analysis
Introduction to Difference in Differences (DID) Analysis Hsueh-Sheng Wu CFDR Workshop Series June 15, 2020 1 Outline of Presentation • What is Difference-in-Differences (DID) analysis • Threats to internal and external validity • Compare and contrast three different research designs • Graphic presentation of the DID analysis • Link between regression and DID • Stata -diff- module • Sample Stata codes • Conclusions 2 What Is Difference-in-Differences Analysis • Difference-in-Differences (DID) analysis is a statistic technique that analyzes data from a nonequivalence control group design and makes a casual inference about an independent variable (e.g., an event, treatment, or policy) on an outcome variable • A non-equivalence control group design establishes the temporal order of the independent variable and the dependent variable, so it establishes which variable is the cause and which one is the effect • A non-equivalence control group design does not randomly assign respondents to the treatment or control group, so treatment and control groups may not be equivalent in their characteristics and reactions to the treatment • DID is commonly used to evaluate the outcome of policies or natural events (such as Covid-19) 3 Internal and External Validity • When designing an experiment, researchers need to consider how extraneous variables may threaten the internal validity and external validity of an experiment • Internal validity refers to the extent to which an experiment can establish the causal relation between the independent variable and -

Experimental Design and Data Analysis in Computer Simulation
Journal of Modern Applied Statistical Methods Volume 16 | Issue 2 Article 2 12-1-2017 Experimental Design and Data Analysis in Computer Simulation Studies in the Behavioral Sciences Michael Harwell University of Minnesota - Twin Cities, [email protected] Nidhi Kohli University of Minnesota - Twin Cities Yadira Peralta University of Minnesota - Twin Cities Follow this and additional works at: http://digitalcommons.wayne.edu/jmasm Part of the Applied Statistics Commons, Social and Behavioral Sciences Commons, and the Statistical Theory Commons Recommended Citation Harwell, M., Kohli, N., & Peralta, Y. (2017). Experimental Design and Data Analysis in Computer Simulation Studies in the Behavioral Sciences. Journal of Modern Applied Statistical Methods, 16(2), 3-28. doi: 10.22237/jmasm/1509494520 This Invited Article is brought to you for free and open access by the Open Access Journals at DigitalCommons@WayneState. It has been accepted for inclusion in Journal of Modern Applied Statistical Methods by an authorized editor of DigitalCommons@WayneState. Journal of Modern Applied Statistical Methods Copyright © 2017 JMASM, Inc. November 2017, Vol. 16, No. 2, 3-28. ISSN 1538 − 9472 doi: 10.22237/jmasm/1509494520 Experimental Design and Data Analysis in Computer Simulation Studies in the Behavioral Sciences Michael Harwell Nidhi Kohli Yadira Peralta University of Minnesota, University of Minnesota, University of Minnesota, Twin Cities Twin Cities Twin Cities Minneapolis, MN Minneapolis, MN Minneapolis, MN Treating computer simulation studies as statistical sampling experiments subject to established principles of experimental design and data analysis should further enhance their ability to inform statistical practice and a program of statistical research. Latin hypercube designs to enhance generalizability and meta-analytic methods to analyze simulation results are presented.