6.10 Biological Fluxes in the Ocean and Atmospheric Pco2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Four Hundred Million Years of Silica Biomineralization in Land Plants
Four hundred million years of silica biomineralization in land plants Elizabeth Trembath-Reicherta,1, Jonathan Paul Wilsonb, Shawn E. McGlynna,c, and Woodward W. Fischera aDivision of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125; bDepartment of Biology, Haverford College, Haverford, PA 19041; and cGraduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji-shi, Tokyo 192-0397, Japan Edited by Thure E. Cerling, University of Utah, Salt Lake City, UT, and approved February 20, 2015 (received for review January 7, 2015) Biomineralization plays a fundamental role in the global silicon Silica is widely used within plants for structural support and cycle. Grasses are known to mobilize significant quantities of Si in pathogen defense (19–21), but it remains a poorly understood the form of silica biominerals and dominate the terrestrial realm aspect of plant biology. Recent work on the angiosperm Oryza today, but they have relatively recent origins and only rose to sativa demonstrated that silica accumulation is facilitated by taxonomic and ecological prominence within the Cenozoic Era. transmembrane proteins expressed in root cells (21–24). Phy- This raises questions regarding when and how the biological silica logenetic analysis revealed that these silicon transport proteins cycle evolved. To address these questions, we examined silica were derived from a diverse family of modified aquaporins that abundances of extant members of early-diverging land plant include arsenite and glycerol transporters (19, 21, 25, 26). A clades, which show that silica biomineralization is widespread different member of this aquaporin family was recently identi- across terrestrial plant linages. Particularly high silica abundances fied that enables silica uptake in the horsetail Equisetum,an are observed in lycophytes and early-diverging ferns. -

Coccolith Dissolution Within Copepod Guts Affects Fecal Pellet Density And
www.nature.com/scientificreports OPEN Coccolith dissolution within copepod guts afects fecal pellet density and sinking rate Received: 17 January 2018 Meredith M. White1,2, Jesica D. Waller1, Laura C. Lubelczyk1, David T. Drapeau1, Accepted: 13 June 2018 Bruce C. Bowler1, William M. Balch1 & David M. Fields1 Published: xx xx xxxx The most common biomineral produced in the contemporary ocean is calcium carbonate, including the polymorph calcite produced by coccolithophores. The surface waters of the ocean are supersaturated with respect to calcium carbonate. As a result, particulate inorganic carbon (PIC), such as calcite coccoliths, is not expected thermodynamically to dissolve in waters above the lysocline (~4500– 6000 m). However, observations indicate that up to 60–80% of calcium carbonate is lost in the upper 500–1000 m of the ocean. This is hypothesized to occur in microenvironments with reduced saturation states, such as zooplankton guts. Using a new application of the highly precise 14C microdifusion technique, we show that following a period of starvation, up to 38% of ingested calcite dissolves in copepod guts. After continued feeding, our data show the gut becomes increasingly bufered, which limits further dissolution; this has been termed the Tums hypothesis (after the drugstore remedy for stomach acid). As less calcite dissolves in the gut and is instead egested in fecal pellets, the fecal pellet sinking rates double, with corresponding increases in pellet density. Our results empirically demonstrate that zooplankton guts can facilitate calcite dissolution above the chemical lysocline, and that carbon export through fecal pellet production is variable, based on the feeding history of the copepod. -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
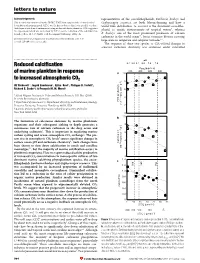
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Silicate Weathering in Anoxic Marine Sediment As a Requirement for Authigenic Carbonate Burial T
Earth-Science Reviews 200 (2020) 102960 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Silicate weathering in anoxic marine sediment as a requirement for authigenic carbonate burial T Marta E. Torresa,*, Wei-Li Hongb, Evan A. Solomonc, Kitty Millikend, Ji-Hoon Kime, James C. Samplef, Barbara M.A. Teichertg, Klaus Wallmannh a College of Earth, Ocean and Atmospheric Science, Oregon State University, Corvallis OR 97331, USA b Geological Survey of Norway, Trondheim, Norway c School of Oceanography, University of Washington, Seattle, WA 98195, USA d Bureau of Economic Geology, University of Texas at Austin, Austin, TX 78713, USA e Petroleum and Marine Research Division, Korea Institute of Geoscience and Mineral Resources, Daejeon, South Korea f School of Earth and Sustainability, Northern Arizona University, Flagstaff, AZ 86011, USA g Geologisch-Paläontologisches Institut, Wilhelms-Universität Münster, Corrensstr. 24, 48149 Münster, Germany h IFM-GEOMAR Leibniz Institute of Marine Sciences, Wischhofstrasse 1-3, 24148 Kiel, Germany ARTICLE INFO ABSTRACT Keywords: We emphasize the importance of marine silicate weathering (MSiW) reactions in anoxic sediment as funda- Silicate weathering mental in generating alkalinity and cations needed for carbonate precipitation and preservation along con- Authigenic carbonate tinental margins. We use a model that couples thermodynamics with aqueous geochemistry to show that the CO2 Organogenic dolomite released during methanogenesis results in a drop in pH to 6.0; unless these protons are buffered by MSiW, Alkalinity carbonate minerals will dissolve. We present data from two regions: the India passive margin and the active Carbon cycling subduction zone off Japan, where ash and/or rivers supply the reactive silicate phase, as reflected in strontium isotope data. -

The Silicate Structure Analysis of Hydrated Portland Cement Paste
The Silicate Structure Analysis of Hydrated Portland Cement Paste CHARLES W. LENTZ, Research Department, Dow Corning Corporation, Midland, Michigan A new technique for recovering silicate structures as trimeth- ylsilyl derivatives has been used to study the hydration of port- land cement. By this method only the changes iii the silicate portion of the structure can be determined as a function of hydration time. Cement pastes ranging in age from one day to 14. 7 years were analyzedfor the study. The hydration reaction is shown to be similar to a condensation type polymerization. The orthosilicate content of cement paste (which probably rep- resents the original calcium silicates in the portland cement) gradually decreases as the paste ages. Concurrentiy a disilicate structure is formed which reaches a maximum quantity in about four weeks and then it too diminishes as the paste ages. Minor quantities of a trisiicate and a cyclic tetrasiicate are shown to be present in hydrated cement paste. An unidentified polysilicate structure is produced by the hydration reaction which not only increases in quantity throughout the age period studied, but also increases in molecular weight as the paste ages. THE SILICON atoms in silicate minerals are always in fourfold coordination with oxygen. These silicon-oxygen tetrahedra can be completely separated from each other, as in the orthosilicates, they can be paired, as in the pyrosilicates, or they can be in other combinations with each other to give a variety of silicate structures. If the min- eral is composed only of Si and 0, then there is a three-dimensional network of silicate tetrahedra. -

Lecture 24 the Oceans Reading: White, Ch 9.1 to 9.7.1 (Or Digital P370-400)
Lecture 24 The Oceans Reading: White, Ch 9.1 to 9.7.1 (or digital p370-400) Last Time 1. The oceans: currents, stratification and chemistry Today 2. the marine carbon cycle GG325 L24, F2013 The Marine Carbon Cycle The marine biosphere and ocean currents control non-conservative chemical signatures in marine water masses. Photosynthetic productivity in the surface waters requires a flux of nutrient- rich waters in to the photic zone. This is variable around the globe. Respirative decomposition of organic matter throughout the marine water column is also variable around the globe. If the oceans did not convect in a semi-steady-state manner, the cycle that controls the concentrations of C, O, N and P in various water masses and the relative biomass that is sustained there would not exist. Today we investigate the movement of carbon in today's oceans. next week we will look to the geologic record to see how past climatic conditions on Earth altered the movement of water masses the motions of carbon through the oceans, plus we consider our future. GG325 L24, F2013 1 Deep Ocean Circulation Effects: The circulation patterns in the 2 oceans that we discussed last time results in what is sometimes referred to as basin-to-basin fractionation. Pacific: deep water inflow is in the south. Return flow is by both upwelling and by diffuse upflow of deep water in the North Pacific. This "in on the bottom out on the top" circulation is like that in most estuaries, thus it is known as estuarine circulation. Atlantic: has deep water formation from This circulation pattern affects nearly every shallow waters: anti- aspect of the marine carbon cycle. -

University of Nevada Reno SILICATE and CARBONATE SEDIMENT
University of Nevada Reno /SILICATE AND CARBONATE SEDIMENT-WATER RELATIONSHIPS IN WALKER LAKE, NEVADA A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Geochemistry by Ronald J. Spencer MINES 2 - UBKAKY %?: The thesis of Ronald James Spencer is approved: University of Nevada Reno May 1977 ACKNOWLEDGMENTS The author gratefully acknowledges the contributions of Dr. L. V. Benson, who directed the thesis, and Drs. L. C. Hsu and R. D. Burkhart, who served on the thesis ccmnittee. A special note of thanks is given to Pat Harris of the Desert Research Institute, who supervised the wet chemical analyses on the lake water and pore fluids. I also would like to thank John Sims and Mike Rymer of the U. S. Geological Survey, Menlo Park, for their effort in obtaining the piston core; and Blair Jones of the U. S. Geological Survey, Reston, for the use of equipment and many very helpful suggestions. Much of the work herein was done as a part of the study of the "Dynamic Ecological Relationships in Walker Lake, Nevada", and was supported by the Office of Water Research and Technology through grant number C-6158 to the Desert Research Institute. I thank the other members involved in the study; Drs. Dave Koch and Roger Jacobson, and Joe Mahoney, Jim Cooper, and Jim Hainiine; for advice in their fields of expertise and help in sample collection. Special thanks are extended to my wife, Laurie, without whose help and support this thesis could not have been completed. A final note of thanks to Tina Nesler, who advised Laurie in the typing of the manuscript. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation. -

Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs
Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs Coral reefs are vital to the long-term to produce CaCO3, carbon dioxide (CO2), As CO2 increases in the atmosphere, viability of coastal societies, providing and water (H2O). Over time as these more is absorbed by the surface of the economic, recreational, and aesthetic organisms grow and die, their skeletons ocean, where it combines with seawater value from which coastal communities break down and become calcium carbon- to make a weak acid called carbonic thrive. Some of the services that coral ate sediments. These sediments fill in acid (H2CO3). This process, called reefs provide include protection from the framework of the reef and eventually ocean acidification, causes a decrease storm waves, nurseries and habitats for become cemented together, construct- in seawater pH (or increase in acidity) commercially important fish species, ing the foundation for continued upward that can result in a decrease in biogenic and production of sand for beaches. growth of the reef structure. The infilling calcification rates, dissolution of carbon- Coral reefs develop over thousands of the reef framework with sediments is ate sediments, and loss of reef structure. of years as tropical marine organ- what allows vertical accretion over time One of the primary concerns associated isms build skeletons of calcium carbonate and enables reef growth to keep up with with ocean acidification is whether coral (CaCO3) minerals to form a three-dimen- sea-level rise. Calcification is a revers- reefs will be able to continue to grow at sional structure (fig. 1). This process, ible process. -

1 Supplementary Materials and Methods 1 S1 Expanded
1 Supplementary Materials and Methods 2 S1 Expanded Geologic and Paleogeographic Information 3 The carbonate nodules from Montañez et al., (2007) utilized in this study were collected from well-developed and 4 drained paleosols from: 1) the Eastern Shelf of the Midland Basin (N.C. Texas), 2) Paradox Basin (S.E. Utah), 3) Pedregosa 5 Basin (S.C. New Mexico), 4) Anadarko Basin (S.C. Oklahoma), and 5) the Grand Canyon Embayment (N.C. Arizona) (Fig. 6 1a; Richey et al., (2020)). The plant cuticle fossils come from localities in: 1) N.C. Texas (Lower Pease River [LPR], Lake 7 Kemp Dam [LKD], Parkey’s Oil Patch [POP], and Mitchell Creek [MC]; all representing localities that also provided 8 carbonate nodules or plant organic matter [POM] for Montañez et al., (2007), 2) N.C. New Mexico (Kinney Brick Quarry 9 [KB]), 3) S.E. Kansas (Hamilton Quarry [HQ]), 4) S.E. Illinois (Lake Sara Limestone [LSL]), and 5) S.W. Indiana (sub- 10 Minshall [SM]) (Fig. 1a, S2–4; Richey et al., (2020)). These localities span a wide portion of the western equatorial portion 11 of Euramerica during the latest Pennsylvanian through middle Permian (Fig. 1b). 12 13 S2 Biostratigraphic Correlations and Age Model 14 N.C. Texas stratigraphy and the position of pedogenic carbonate samples from Montañez et al., (2007) and cuticle were 15 inferred from N.C. Texas conodont biostratigraphy and its relation to Permian global conodont biostratigraphy (Tabor and 16 Montañez, 2004; Wardlaw, 2005; Henderson, 2018). The specific correlations used are (C. Henderson, personal 17 communication, August 2019): (1) The Stockwether Limestone Member of the Pueblo Formation contains Idiognathodus 18 isolatus, indicating that the Carboniferous-Permian boundary (298.9 Ma) and base of the Asselian resides in the Stockwether 19 Limestone (Wardlaw, 2005).