Of 67 the Diagrams Show Two Different Models of an Atom. 'Plum Pudding'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Radiochemistry of Beryllium
National Academy of Sciences National Research Council I NUCLEAR SCIENCE SERIES The Radiochemistry ·of Beryllium COMMITTEE ON NUCLEAR SCIENCE L. F. CURTISS, Chairman ROBLEY D. EVANS, Vice Chairman National Bureau of Standards MassaChusetts Institute of Technol0gy J. A. DeJUREN, Secretary ./Westinghouse Electric Corporation H.J. CURTIS G. G. MANOV Brookhaven National' LaboratOry Tracerlab, Inc. SAMUEL EPSTEIN W. WAYNE MEINKE CalUornia Institute of Technology University of Michigan HERBERT GOLDSTEIN A.H. SNELL Nuclear Development Corporation of , oak Ridge National Laboratory America E. A. UEHLING H.J. GOMBERG University of Washington University of Michigan D. M. VAN PATTER E.D.KLEMA Bartol Research Foundation Northwestern University ROBERT L. PLATZMAN Argonne National Laboratory LIAISON MEMBERS PAUL C .. AEBERSOLD W.D.URRY Atomic Energy Commission U. S. Air Force J. HOW ARD McMILLEN WILLIAM E. WRIGHT National Science Foundation Office of Naval Research SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chairman HAROLD KIRBY University of Michigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE Florida State University. Oak Ridge National Laboratory GEORGE A. COW AN JULIAN NIELSEN Los Alamos Scientific Laboratory Hanford Laboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG University of Washington Argonne National Laboratory JEROME HUDIS PETER C. STEVENSON Brookhaven National Laboratory University of California (Livermore) EARL HYDE LEO YAFFE University of CalUornia (Berkeley) McGill University CONSULTANTS NATHAN BALLOU WILLIAM MARLOW Naval Radiological Defense Laboratory N atlonal Bureau of Standards JAMESDeVOE University of Michigan CHF.MISTRY-RADIATION AND RADK>CHEMIST The Radiochemistry of Beryllium By A. W. FAIRHALL. Department of Chemistry University of Washington Seattle, Washington May 1960 ' Subcommittee on Radiochemistry National Academy of Sciences - National Research Council Printed in USA. -

Volatility of Radiopharmacy-Prepared Sodium Iodide-131 Capsules
RADIATION SAFETY Volatility of Radiopharmacy-Prepared Sodium Iodide-131 Capsules James M. Bright, Trenton T. Rees, Louis E. Baca and Richard L. Green Pharmacy Services, Syncor International Corporation, Albuquerque, New Mexico; and Quality and Regulatory Department, Syncor International Corporation, Woodland Hills, California studied thyroid physiology using 128I in 1937 (1,2). In January Objective: The aims of this study were to quantify the extent 1941, Hertz and Roberts were the first to administer radioiodine of volatilization from 131I-NaI therapeutic capsules prepared in 131I for the treatment of hyperthyroid patients (3). Today, almost a centralized radiopharmacy and to quantify the amount of 60 y later, radioiodine therapy with 131I remains the primary volatile 131I released from a dispensing vial containing a compounded 131I-NaI therapy capsule. therapeutic agent used in nuclear medicine, and its use is firmly Methods: Therapy capsules were prepared by injecting 131I established in the 2 diseases first treated: hyperthyroidism and oral solution into capsules containing anhydrous dibasic thyroid carcinoma. sodium phosphate. Volatilized activity was obtained by filter- Initially the use of 131I was restricted to the only pharmaceu- ing air drawn across samples that were placed open on the tical dosage form then available—liquid oral solution. While 131 bottom of a sample holder cup. Volatile I was captured by liquid radioiodine proved to be beneficial to the patients to filtering it through 3 triethylenediamine-impregnated carbon whom it was administered, the frequency of contamination and cartridge filters, arranged in series. To quantify the amount of thyroid uptake activity in nuclear medicine personnel who volatile 131I released from a dispensing vial during a simulated patient administration, a vial containing a compounded 131I handled the material was noted with increasing alarm (4–7). -

Measurement of 10Be from Lake Malawi (Africa) Drill Core Sediments and Implications for Geochronology
Palaeogeography, Palaeoclimatology, Palaeoecology 303 (2011) 110–119 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Measurement of 10Be from Lake Malawi (Africa) drill core sediments and implications for geochronology L.R. McHargue a,⁎, A.J.T. Jull b, A. Cohen a a Department of Geosciences, University of Arizona, 1040 E. Fourth Street, Tucson, Arizona 85721, United States b NSF Arizona AMS Laboratory, University of Arizona, 1118 E. Fourth Street, Tucson, Arizona 85721, United States article info abstract Article history: The cosmogenic radionuclide 10Be was measured from drill core sediments from Lake Malawi in order to Received 19 August 2008 help construct a chronology for the study of the tropical paleoclimate in East Africa. Sediment samples were Received in revised form 29 January 2010 taken every 10 m from the core MAL05-1C to 80 m in depth and then from that depth in core MAL05-1B to Accepted 4 February 2010 382 m. Sediment samples were then later taken at a higher resolution of every 2 m from MAL05-1C. They Available online 12 February 2010 were then leached to remove the authigenic fraction, the leachate was processed to separate out the beryllium isotopes, and 10Be was measured at the TAMS Facility at the University of Arizona. The 10Be/9Be Keywords: fi Beryllium-10 pro le from Lake Malawi sediments is similar to those derived from marine sediment cores for the late Cosmogenic radionuclide Pleistocene, and is consistent with the few radiocarbon and OSL IR measurements made from the same core. Lake sediments Nevertheless, a strong correlation between the stable isotope 9Be and the cosmogenic isotope 10Be suggests Lake Malawi that both isotopes have been well mixed before deposition unlike in some marine sediment cores. -

Meteoric Be and Be As Process Tracers in the Environment
Chapter 5 Meteoric 7Be and 10Be as Process Tracers in the Environment James M. Kaste and Mark Baskaran 7 10 Abstract Be (T1/2 ¼ 53 days) and Be (T1/2 ¼ occurring Be isotopes of use to Earth scientists are the 7 1.4 Ma) form via natural cosmogenic reactions in the short-lived Be (T1/2 ¼ 53.1 days) and the longer- 10 atmosphere and are delivered to Earth’s surface by wet lived Be (T1/2 ¼ 1.4 Ma; Nishiizumi et al. 2007). and dry deposition. The distinct source term and near- Because cosmic rays that cause the initial cascade of constant fallout of these radionuclides onto soils, vege- neutrons and protons in the upper atmosphere respon- tation, waters, ice, and sediments makes them valuable sible for the spallation reactions are attenuated by tracers of a wide range of environmental processes the mass of the atmosphere itself, production rates of operating over timescales from weeks to millions of comsogenic Be are three orders of magnitude higher in years. Beryllium tends to form strong bonds with oxygen the stratosphere than they are at sea-level (Masarik and atoms, so 7Be and 10Be adsorb rapidly to organic and Beer 1999, 2009). Most of the production of cosmo- inorganic solid phases in the terrestrial and marine envi- genic Be therefore occurs in the upper atmosphere ronment. Thus, cosmogenic isotopes of beryllium can be (5–30 km), although there is trace, but measurable used to quantify surface age, sediment source, mixing production as oxygen atoms in minerals at the Earth’s rates, and particle residence and transit times in soils, surface are spallated (in situ produced; see Lal 2011, streams, lakes, and the oceans. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom
Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom Democritus- Greek Philosopher proposed that if something was divided enough times, eventually the particles would be too small to divide any further. Ex: Identify this Greek philosopher who postulated that if an object was divided enough times, there would eventually be small particles that could not be divided any further. Discoverers of the Atom John Dalton- English chemist who made the “billiard ball” atom model. First to prove that rainfall was a result of temperature change. He was the first scientist after Democritus to build on atomic theory. He also created a law on partial pressures. Common Clues: Partial pressures, pioneer of atomic theory, and temperature change causes rainfall. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. Discoverers of the Atom J.J. Thomson- English Scientist who discovered electrons through a cathode. Made the “plum pudding model” with Lord Kelvin (Kelvin Scale) which stated that negative charges were spread about a positive charged medium, making atoms neutral. Common Clues: Plum pudding, Electrons had negative charges, disproved by either Rutherford or Mardsen and Geiger The image cannot be displayed. -

Activation Analysis
26 August 1967 Leading Articles MEDIBALJOURNAL 509 her patients or the hospital midwife to undertake the home lation has been found between arsenic level in the blood and visiting of them after discharge. degree of renal insufficiency as indicated by blood creatinine Br Med J: first published as 10.1136/bmj.3.5564.509 on 26 August 1967. Downloaded from From the Bradford reports it can be seen that the final measurements. But speculative inquiries of this kind are decision to permit a mother and baby home after 48 hours worth while because they may sometimes provide the first must be made by someone at least of registrar status. It clue to the solution of an intractable problem. Information would also be of considerable value if the general practitioner of a more immediately useful kind can be expected from and district midwife in charge of such a case could freely using activation analysis in problems where the underlying obtain an opinion by domiciliary consultation from either the biochemical and physiological considerations are better hospital obstetric or paediatric department on any puerperal understood. or neonatal complication before readmission of the woman Thyroid metabolism, much studied by radioactive isotopes, after she has returned home. offers interesting problems for attack by activation analysis. When early discharge was discussed in these columns three Semi-automatic methods for the routine determination of years ago' the conclusion was drawn that, though it might protein-bound stable iodine have been elaborated, notably be suitable in emergency conditions, it had little part in in France,2 where the establishment of a laboratory to carry long-term planning. -
![Arxiv:1708.07449V1 [Nucl-Ex] 24 Aug 2017](https://docslib.b-cdn.net/cover/1448/arxiv-1708-07449v1-nucl-ex-24-aug-2017-911448.webp)
Arxiv:1708.07449V1 [Nucl-Ex] 24 Aug 2017
August 25, 2017 0:18 WSPC/INSTRUCTION FILE cosmogenics-ijmpa International Journal of Modern Physics A c World Scientific Publishing Company Cosmogenic activation of materials Susana Cebri´an Grupo de F´ısica Nuclear y Astropart´ıculas, Universidad de Zaragoza, Calle Pedro Cerbuna 12, 50009 Zaragoza, Spain Laboratorio Subterr´aneo de Canfranc, Paseo de los Ayerbe s/n 22880 Canfranc Estaci´on, Huesca, Spain [email protected] Received Day Month Year Revised Day Month Year Experiments looking for rare events like the direct detection of dark matter particles, neutrino interactions or the nuclear double beta decay are operated deep underground to suppress the effect of cosmic rays. But the production of radioactive isotopes in ma- terials due to previous exposure to cosmic rays is an hazard when ultra-low background conditions are required. In this context, the generation of long-lived products by cosmic nucleons has been studied for many detector media and for other materials commonly used. Here, the main results obtained on the quantification of activation yields on the Earth’s surface will be summarized, considering both measurements and calculations following different approaches. The isotope production cross sections and the cosmic ray spectrum are the two main ingredients when calculating this cosmogenic activation; the different alternatives for implementing them will be discussed. Activation that can take place deep underground mainly due to cosmic muons will be briefly commented too. Presently, the experimental results for the cosmogenic production of radioisotopes are scarce and discrepancies between different calculations are important in many cases, but the increasing interest on this background source which is becoming more and more relevant can help to change this situation. -
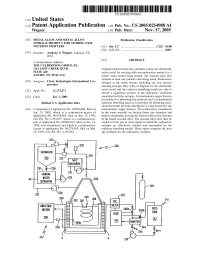
(12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub
US 2005O254988A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub. Date: Nov. 17, 2005 (54) METALALLOY AND METALALLOY Publication Classification STORAGE PRODUCT FOR STORING EAST NEUTRON EMITTERS (51) Int. Cl. ................................................. C22C 43/00 (52) U.S. Cl. .................................................................. 420/1 (75) Inventor: Anthony S. Wagner, Lakeway, TX (US) (57) ABSTRACT Correspondence Address: THE CULBERTSON GROUP, PC. 1114 LOST CREEK BLVD. Aliquid reactant metal alloy includes at least one chemically SUTE 420 active metal for reacting with non-radioactive material in a AUSTIN, TX 78746 (US) mixed waste Stream being treated. The reactant alloy also includes at least one radiation absorbing metal. Radioactive (73) Assignee: Clean Technologies International Cor isotopes in the waste Stream, including any fast neutron poration emitting isotopes alloy with, or disperse in, the chemically Appl. No.: 11/173,271 active metal and the radiation absorbing metals are able to (21) absorb a significant portion of the radioactive emissions (22) Filed: Jul. 1, 2005 asSociated with the isotopes. A transmutation target fraction is included for absorbing fast neutrons and a transmutation Related U.S. Application Data emission absorbing fraction is provided for absorbing emis sions that result from the absorption of a fast neutron by the (63) Continuation of application No. 10/059,808, filed on transmutation target fraction. Non-radioactive constituents Jan. 29, 2002, which is a continuation-in-part of in the waste material are broken down into harmless and application No. 09/334,985, filed on Jun. 17, 1999, useful constituents, leaving the alloyed radioactive isotopes now Pat. -

Lecture #3, Atomic Structure (Rutherford, Bohr Models)
Welcome to 3.091 Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr Periodic Table Quiz 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 Name Grade /10 Image by MIT OpenCourseWare. La Lazy Ce college Pr professors Nd never Pm produce Sm sufficiently Eu educated Gd graduates Tb to Dy dramatically Ho help Er executives Tm trim Yb yearly Lu losses. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. La Loony Ce chemistry Pr professor Nd needs Pm partner: Sm seeking cannot be referring Eu educated to 3.091! Gd graduate Tb to must be the “other” Dy develop Ho hazardous chemistry professor Er experiments Tm testing Yb young Lu lab assistants. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. 138.9055 920 57 3455 3 6.146 * 1.10 57 5.577 La [Xe]5d16s2 Lanthanum CEase not I to slave, back breaking to tend; PRideless and bootless stoking hearth and fire. No Dream of mine own precious time to spend Pour'ed More to sate your glutt'nous desire. -

Electron Charge Density: a Clue from Quantum Chemistry for Quantum Foundations
Electron Charge Density: A Clue from Quantum Chemistry for Quantum Foundations Charles T. Sebens California Institute of Technology arXiv v.2 June 24, 2021 Forthcoming in Foundations of Physics Abstract Within quantum chemistry, the electron clouds that surround nuclei in atoms and molecules are sometimes treated as clouds of probability and sometimes as clouds of charge. These two roles, tracing back to Schr¨odingerand Born, are in tension with one another but are not incompatible. Schr¨odinger'sidea that the nucleus of an atom is surrounded by a spread-out electron charge density is supported by a variety of evidence from quantum chemistry, including two methods that are used to determine atomic and molecular structure: the Hartree-Fock method and density functional theory. Taking this evidence as a clue to the foundations of quantum physics, Schr¨odinger'selectron charge density can be incorporated into many different interpretations of quantum mechanics (and extensions of such interpretations to quantum field theory). Contents 1 Introduction2 2 Probability Density and Charge Density3 3 Charge Density in Quantum Chemistry9 3.1 The Hartree-Fock Method . 10 arXiv:2105.11988v2 [quant-ph] 24 Jun 2021 3.2 Density Functional Theory . 20 3.3 Further Evidence . 25 4 Charge Density in Quantum Foundations 26 4.1 GRW Theory . 26 4.2 The Many-Worlds Interpretation . 29 4.3 Bohmian Mechanics and Other Particle Interpretations . 31 4.4 Quantum Field Theory . 33 5 Conclusion 35 1 1 Introduction Despite the massive progress that has been made in physics, the composition of the atom remains unsettled. J. J. Thomson [1] famously advocated a \plum pudding" model where electrons are seen as tiny negative charges inside a sphere of uniformly distributed positive charge (like the raisins|once called \plums"|suspended in a plum pudding). -

Cbiescss05.Pdf
Science IX Sample Paper 5 Solved www.rava.org.in CLASS IX (2019-20) SCIENCE (CODE 086) SAMPLE PAPER-5 Time : 3 Hours Maximum Marks : 80 General Instructions : (i) The question paper comprises of three sections-A, B and C. Attempt all the sections. (ii) All questions are compulsory. (iii) Internal choice is given in each sections. (iv) All questions in Section A are one-mark questions comprising MCQ, VSA type and assertion-reason type questions. They are to be answered in one word or in one sentence. (v) All questions in Section B are three-mark, short-answer type questions. These are to be answered in about 50-60 words each. (vi) All questions in Section C are five-mark, long-answer type questions. These are to be answered in about 80-90 words each. (vii) This question paper consists of a total of 30 questions. 4. What is the S.I. unit of momentum ? [1] SECTION -A (a) kgms (b) mskg−1 −1 −1 DIRECTION : For question numbers 1 and 2, two statements (c) kgms (d) kg() ms are given- one labelled Assertion (A) and the other labelled Ans : (c) kgms−1 Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below. 5. Which of the following is not a perfectly in elastic collision ? [1] (a) Both A and R are true and R is correct explanation (a) Capture of an electron by proton. of the assertion. (b) Man jumping on to a moving cart. (b) Both A and R are true but R is not the correct explanation of the assertion.