Literature Survey on Predators of Snakes in Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Identification of Shed Snake Skins in Taiwan and Adjacent Islands
Zoological Studies 56: 38 (2017) doi:10.6620/ZS.2017.56-38 Open Access Species Identification of Shed Snake Skins in Taiwan and Adjacent Islands Tein-Shun Tsai1,* and Jean-Jay Mao2 1Department of Biological Science and Technology, National Pingtung University of Science and Technology 1 Shuefu Road, Neipu, Pingtung 912, Taiwan 2Department of Forestry and Natural Resources, National Ilan University No.1, Sec. 1, Shennong Rd., Yilan City, Yilan County 260, Taiwan. E-mail: [email protected] (Received 28 August 2017; Accepted 25 November 2017; Published 19 December 2017; Communicated by Jian-Nan Liu) Tein-Shun Tsai and Jean-Jay Mao (2017) Shed snake skins have many applications for humans and other animals, and can provide much useful information to a field survey. When properly prepared and identified, a shed snake skin can be used as an important voucher; the morphological descriptions of the shed skins may be critical for taxonomic research, as well as studies of snake ecology and conservation. However, few convenient/ expeditious methods or techniques to identify shed snake skins in specific areas have been developed. In this study, we collected and examined a total of 1,260 shed skin samples - including 322 samples from neonates/ juveniles and 938 from subadults/adults - from 53 snake species in Taiwan and adjacent islands, and developed the first guide to identify them. To the naked eye or from scanned images, the sheds of almost all species could be identified if most of the shed was collected. The key features that aided in identification included the patterns on the sheds and scale morphology. -

Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002
Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Kadoorie Farm and Botanic Garden in collaboration with Guangxi Zhuang Autonomous Region Forestry Department Guangxi Forestry Survey and Planning Institute South China Institute of Botany South China Normal University Institute of Zoology, CAS March 2003 South China Forest Biodiversity Survey Report Series: No. 27 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Editors John R. Fellowes, Bosco P.L. Chan, Michael W.N. Lau, Ng Sai-Chit and Gloria L.P. Siu Contributors Kadoorie Farm and Botanic Garden: Gloria L.P. Siu (GS) Bosco P.L. Chan (BC) John R. Fellowes (JRF) Michael W.N. Lau (ML) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Graham T. Reels (GTR) Roger C. Kendrick (RCK) Guangxi Zhuang Autonomous Region Forestry Department: Xu Zhihong (XZH) Pun Fulin (PFL) Xiao Ma (XM) Zhu Jindao (ZJD) Guangxi Forestry Survey and Planning Institute (Comprehensive Tan Wei Fu (TWF) Planning Branch): Huang Ziping (HZP) Guangxi Natural History Museum: Mo Yunming (MYM) Zhou Tianfu (ZTF) South China Institute of Botany: Chen Binghui (CBH) Huang Xiangxu (HXX) Wang Ruijiang (WRJ) South China Normal University: Li Zhenchang (LZC) Chen Xianglin (CXL) Institute of Zoology CAS (Beijing): Zhang Guoqing (ZGQ) Chen Deniu (CDN) Nanjing University: Chen Jianshou (CJS) Wang Songjie (WSJ) Xinyang Teachers’ College: Li Hongjing (LHJ) Voluntary specialist: Keith D.P. Wilson (KW) Background The present report details the findings of visits to Northwest Guangxi by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme. -

Ophiophagy and Egg-Eating in Mannophryne Cf. Trinitatis
©Österreichische Gesellschaft für Herpetologie e.V., Wien, Austria, download unter www.biologiezentrum.at SHORT NOTE HERPETOZOA 18 (1/2) Wien, 30. Juni 2005 SHORT NOTE 69 Ophiophagy and egg-eating in Predator specimens of the egg-eating case Mannophryne cf. trinitatis were deposited in the herpetological collec- tion of the "Laboratorio de Biogeografìa, (GARMAN, 1888) Universidad de Los Andes, Mérida, Venezu- Amphibians are considered to be feed- ela (ULABG). Digital photos were taken ing opportunists with their diets reflecting with a Sony Mavica® camera. Poor resolu- the availability of food of appropriate size tion pictures for printing were used to make which seems to be a basic constraint in ink-draw pictures at scale, and original pho- amphibian diet (DUELLMAN & TRUEB 1986). tographic documentation was deposited in As to cases of snake-eating (ophiophagy), the "Museo del Intituto de Zoologia Agrico- Leptodactylus pentadactylus (LAURENTI, la" (MIZA), "Universidad Central de Vene- 1768) has been reported to predate on adult zuela". Technical equipment for measure- Imantodes cenchoa (LINNAEUS, 1758), Rana ments: GPS Garmin® 12, Texas Instruments® catesbeiana SHAW, 1802 on Micrurus, Pyxi- digital thermohygrometer, Casio® digital alti- cephalus adspersus TSCHUDI, 1838 on He- meter, Mitutoyo® digital caliper for lengths machatus and some Malaysian toads of the < 30 mm, and a calibrated ruler for lengths genus Bufo commonly feed on Flowerpot > 30 mm. Blind Snakes, Rhamphotyphlops braminus On May 7th, 2002, between 10:15 and (DAUDIN, 1803) (GREEN 1997). Dendro- 12:35 after heavy rainfall, we watched M. batid diet has been studied for some species cf. trinitatis at a stream located at 10°43'N; (e.g., PRADERIO 1985; PINERO & DURANT 62°48'W, at an elevation of 186 m a.s.l., 1993; PINERO & LA MARCA 1995), however, approximately 4.2 km airline from San Juan to our knowledge, no case of ophiophagy de Las Galdonas (Muncipio Arismendi) on has been reported for members of the fami- the road to Aroa, in Sucre State, Venezuela. -
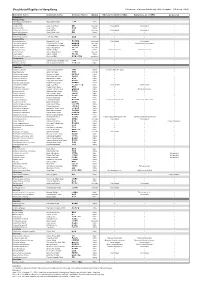
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

P. 1 AC27 Inf. 7 (English Only / Únicamente En Inglés / Seulement
AC27 Inf. 7 (English only / únicamente en inglés / seulement en anglais) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Species trade and conservation IUCN RED LIST ASSESSMENTS OF ASIAN SNAKE SPECIES [DECISION 16.104] 1. The attached information document has been submitted by IUCN (International Union for Conservation of * Nature) . It related to agenda item 19. * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat or the United Nations Environment Programme concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. AC27 Inf. 7 – p. 1 Global Species Programme Tel. +44 (0) 1223 277 966 219c Huntingdon Road Fax +44 (0) 1223 277 845 Cambridge CB3 ODL www.iucn.org United Kingdom IUCN Red List assessments of Asian snake species [Decision 16.104] 1. Introduction 2 2. Summary of published IUCN Red List assessments 3 a. Threats 3 b. Use and Trade 5 c. Overlap between international trade and intentional use being a threat 7 3. Further details on species for which international trade is a potential concern 8 a. Species accounts of threatened and Near Threatened species 8 i. Euprepiophis perlacea – Sichuan Rat Snake 9 ii. Orthriophis moellendorfi – Moellendorff's Trinket Snake 9 iii. Bungarus slowinskii – Red River Krait 10 iv. Laticauda semifasciata – Chinese Sea Snake 10 v. -

List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Cheloniidae 4 Loggerhead Turtle Caretta caretta Green Turtle Chelonia mydas Hawksbill Turtle Eretmochelys imbricata Olive Ridley Turtle Lepidochelys olivacea Dermochelyidae 1 Leatherback Turtle Dermochelys coriacea Emydidae 1 Red-eared Slider * Trachemys scripta elegans Geoemydidae 3 Three-banded Box Turtle Cuora trifasciata Reeves' Turtle Mauremys reevesii Beale's Turtle Sacalia bealei Platysternidae 1 Big-headed Turtle Platysternon megacephalum Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Agamidae 1 Changeable Lizard Calotes versicolor Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Long-tailed Skink Eutropis longicaudata Chinese Skink Plestiodon chinensis chinensis Five-striped Blue-tailed Skink Plestiodon elegans Blue-tailed Skink Plestiodon quadrilineatus Vietnamese Five-lined Skink Plestiodon tamdaoensis Slender Forest Skink Scincella modesta Reeve's Smooth Skink Scincella reevesii Brown Forest Skink Sphenomorphus incognitus Indian Forest Skink Sphenomorphus indicus Chinese Waterside Skink Tropidophorus sinicus Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek's Burrowing Lizard Dibamus bogadeki Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko Gekko gecko Bowring's Gecko Hemidactylus bowringii Brook's Gecko* Hemidactylus brookii House Gecko* Hemidactylus -

Effects of Restoration Habitats on Snake Species of Dghoumes National Park (Tunisia)
Biodiversity Journal , 2019, 10 (3): 213–220 https://doi.org/10.31396/Biodiv.Jour.2019.10.3.213.220 Effects of restoration habitats on snake species of Dghoumes National Park (Tunisia) Ernesto Filippi Via Aurelia 18, 00040 Ariccia (Rome) Italy; e-mail: [email protected] ABSTRACT Dghoumes National Park (DNP) is an important protected area located in South Tunisia. This region is characterized by desertification and habitat degradation, mainly caused by climate changes and overgrazing by domestic and semi-domestic livestock. Habitat regeneration, pro - tection of native wildlife and reintroduction of some animal species are at the centre of the activity of this protected area. This article summarizes the results of the first study carried out on the snakes inhabiting this National Park. The study aimed at working out the first checklist of the species and at making a preliminary analysis of the consequences of habitat restoration on the snake species inside the National Park. KEY WORDS Desertification; habitat alteration; habitat restoration; Snakes; Tunisia. Received 19.05.2019; accepted 02.07.2019; published online 20.08.2019. INTRODUCTION close to Tozeur (Fig. 1), in a territory affected by desertification and habitat degradation, mainly Desertification, the process of semiarid ecosys - caused by climate changes and overgrazing by do - tem deterioration, including drought, soil degrada - mestic and semi-domestic livestock (goats, sheep tion and vegetation loss in arid environments, and dromedaries in a semi-wild state) (Woodfine et covers a wide variety of interactive phenomena, al., 2009). DNP protects a small (7,000 ha), but re - both natural and anthropogenic (Hillel & Rosen - markably diverse, remnant of sub-desertic continen - zweig, 2002). -

Negative Impact of an Invasive Small Indian Mongoose Herpestes
117 Negative Impact of an Invasive Small Indian Mongoose Herpestes javanicus on Native Wildlife Species and Evaluation of a Control Project in Amami-Ohshima and Okinawa Islands, Japan 1 2 Fumio YAMADA * and Ken SUGIMURA 1Department of Wildlife Biology, Forestry and Forest Products Research Institute (FFPRI), PO Box 16, Tsukuba-Norin, Ibaraki 305-8687, Japan e-mail: [email protected] 2Department of Forest of Management, FFPRI, PO Box 16, Tsukuba-Norin, Ibaraki 305-8687, Japan *corresponding author Abstract The small Indian mongoose, Herpestes javanicus (Family Herpestidae, Order Carnivora, Mammalia), is one of the most urgent species to eradicate among invasive mammals in Japan. Recently, the Japanese government has begun full-scale control of the mongoose as a model case for conserving the biodiversity of Japan’s subtropical islands. We review and assess the impacts of the mongoose and control practices on Amami-Ohshima Island and Okinawa Island based on recently acquired information. On Amami-Ohshima Island, a total of 9,960 mongooses were captured under the full-scale project of the Ministry of the Environment which ran for four years from 1999 to October 2003. According to the April 2002 mongoose census taken after three years of full-scale implementation, the population was estimated to be 1/4 (1,500-2,000 mongooses) less than in 1999. However, our investigation revealed that the remaining mongooses in the mountain- ous area are having negative impacts on native species, especially on the Amami rabbit, Pentalagus furnessi. People who are involved with the full-scale mongoose project should recognize that the investment in the project, at least regarding the number of traps and trapping period, is incredibly low at this stage and funding should be increased to achieve the goal. -

Snake Communities Worldwide
Web Ecology 6: 44–58. Testing hypotheses on the ecological patterns of rarity using a novel model of study: snake communities worldwide L. Luiselli Luiselli, L. 2006. Testing hypotheses on the ecological patterns of rarity using a novel model of study: snake communities worldwide. – Web Ecol. 6: 44–58. The theoretical and empirical causes and consequences of rarity are of central impor- tance for both ecological theory and conservation. It is not surprising that studies of the biology of rarity have grown tremendously during the past two decades, with particular emphasis on patterns observed in insects, birds, mammals, and plants. I analyse the patterns of the biology of rarity by using a novel model system: snake communities worldwide. I also test some of the main hypotheses that have been proposed to explain and predict rarity in species. I use two operational definitions for rarity in snakes: Rare species (RAR) are those that accounted for 1% to 2% of the total number of individuals captured within a given community; Very rare species (VER) account for ≤ 1% of individuals captured. I analyse each community by sample size, species richness, conti- nent, climatic region, habitat and ecological characteristics of the RAR and VER spe- cies. Positive correlations between total species number and the fraction of RAR and VER species and between sample size and rare species in general were found. As shown in previous insect studies, there is a clear trend for the percentage of RAR and VER snake species to increase in species-rich, tropical African and South American commu- nities. This study also shows that rare species are particularly common in the tropics, although habitat type did not influence the frequency of RAR and VER species. -

Human-Snake Conflict Patterns in a Dense Urban-Forest Mosaic Landscape
Herpetological Conservation and Biology 14(1):143–154. Submitted: 9 June 2018; Accepted: 25 February 2019; Published: 30 April 2019. HUMAN-SNAKE CONFLICT PATTERNS IN A DENSE URBAN-FOREST MOSAIC LANDSCAPE SAM YUE1,3, TIMOTHY C. BONEBRAKE1, AND LUKE GIBSON2 1School of Biological Sciences, University of Hong Kong, Pokfulam, Hong Kong, China 2School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen, China 3Corresponding author, e-mail: [email protected] Abstract.—Human expansion and urbanization have caused an escalation in human-wildlife conflicts worldwide. Of particular concern are human-snake conflicts (HSC), which result in over five million reported cases of snakebite annually and significant medical costs. There is an urgent need to understand HSC to mitigate such incidents, especially in Asia, which holds the highest HSC frequency in the world and the highest projected urbanization rate, though knowledge of HSC patterns is currently lacking. Here, we examined the relationships between season, weather, and habitat type on HSC incidents since 2002 in Hong Kong, China, which contains a mixed landscape of forest, dense urban areas, and habitats across a range of human disturbance and forest succession. HSC frequency peaked in the autumn and spring, likely due to increased activity before and after winter brumation. There were no considerable differences between incidents involving venomous and non-venomous species. Dense urban areas had low HSC, likely due to its inhospitable environment for snakes, while forest cover had no discernible influence on HSC. We found that disturbed or lower quality habitats such as shrubland or areas with minimal vegetation had the highest HSC, likely because such areas contain intermediate densities of snakes and humans, and intermediate levels of disturbance. -

A New Species of the Genus Achalinus from Huangshan, Anhui, China (Squamata: Xenodermidae)
ORIGINAL Asian Herpetological Research 2021, 12(2): xxx–xxx ARTICLE DOI: 10.16373/j.cnki.ahr.200075 A New Species of the Genus Achalinus from Huangshan, Anhui, China (Squamata: Xenodermidae) Ruyi HUANG1,2,3#, Lifang PENG1,3#, Lei YU4, Tianqi HUANG5, Ke JIANG6, Li DING6, Jinkang CHANG7, Diancheng YANG1,3, Yuhao XU8 and Song HUANG1,3* 1 Anhui Province Key Laboratory of the Conservation and Exploitation of Biological Resource, College of Life Sciences, Anhui Normal University, Wuhu 241000, Anhui, China 2 Shanghai Jian Qiao University, Shanghai 201306, China 3 Huangshan Noah Biodiversity Institute, Huangshan 245000, Anhui, China 4 Anhui Rare Birds Protection Association, Hefei 230601, Anhui, China 5 Graduate Program in Ecology and Evolution, Department of Ecology, Evolution, and Natural Resources, Rutgers, the State University of New Jersey, New Brunswick, NJ 08901, USA 6 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China 7 School of plant protection, Anhui Agricultural University, Hefei 230036, Anhui, China 8 School of Life Sciences, Anhui Agricultural University, Hefei 230036, Anhui, China 1. Introduction Abstract A new species of the genus Achalinus is described based on five specimens collected from the There are currently 13 described species in the genus Achalinus villages of Huangjialing and Fuxi, Huangshan, Anhui, Peters, 1869 (Serpentes: Xenodermidae): A. ater1, A. emilyae, China. It can be morphologically differentiated A. formosanus2, A. hainanus3, I, A. jinggangensis4, II, A. juliani, A. from all the other species in Achalinus except for A. meiguensis5, III, A. niger6, IV, A. ru fescens7, A. spinalis8, A. timi, A. spinalis and A. werneri by the presence of a dotted werneri, and A. -

Ophiophagy in Coronella Austriaca:� ���� �� �� ��������� �� Hierophis Viridiflavus and First Direct Observations of Predation on Vipera Aspis
Herpetology Notes, volume 13: 1107-1110 (2020) (published online on 28 December 2020) Ophiophagy in Coronella austriaca:� �i�� a� o� p��aion on Hierophis viridiflavus an� �i�� �i�� ob��vaion� o� p��aion on Vipera aspis Matteo Riccardo Di Nicola1,*, Lilli Zecchin2, Maurizio D’Amico3, and Francesco Paolo Faraone4 The smooth snake Coronella austriaca Laurenti, 1768 preyed on only by adults (Brown et al., 2013; Reading is a medium/small-sized colubrid snake (total length and Jofré, 2013). usually up to 70 cm, rarely up to 80 cm [Geniez, 2018]) Coronella austriaca can directly swallow smaller prey with a Euro-Siberian (with Caucasian and Anatolian alive, while killing larger prey by constriction (Bruno extensions) chorotype (Sindaco et al., 2013). It is and Maugeri, 1990; Speybroeck et al., 2016; Geniez, distributed across most of Europe (except for Ireland, 2018). Northern England and Central-Northern Scandinavia) Ophiophagy represents only a small part of the C. and eastwards reaching western Kazakhstan, the austriaca diet (Rugiero et al., 1995; Luiselli et al., 1996; Caucasus, northern Anatolia and northern Iran. The Drobenkov, 2000; Reading and Jofré, 2013; Drobenkov, species is polytypic and two subspecies are currently 2014), but it has been directly observed or detected recognised: C. a. austriaca Laurenti, 1768 and C. a. by stomach and faecal contents on several occasions acutirostris Malkmus, 1995 (Sindaco et al., 2013; (including various episodes of cannibalism). The main Speybroeck et al., 2016; Di Nicola et al., 2019). ophiophagy records available in the literature for the The trophic spectrum is a widely studied aspect in smooth snake are summarised in Table 1.