Addition of Regular Insulin to Ternary Parenteral Nutrition: a Stability Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PROTOCOL: Compassionate Use of Omegaven® IV Fat Emulsion
PROTOCOL: Compassionate Use of Omegaven® IV Fat Emulsion Background In the United States, patients dependent upon parenteral nutrition (PN) receive parenteral fat emulsions composed of soybean oils. Lipids are necessary in PN dependent patients due to their high caloric value and essential fatty acid content. However, intravenous lipid emulsions have been implicated in predisposing patients to PN associated liver disease. Phytosterols such as those contained in soybean oils are thought to have a deleterious effect on biliary secretion. Accumulation of lipids in the hepatic Kupffer cells may further impair liver function. Although the currently available omega-6 fatty acid emulsions prevent fatty acid deficiency, it is thought that they are not cleared in a manner similar to enteral chylomicrons and therefore accumulate in the liver and resulting in steatotic liver injury (neonatal cholestasis). It is hypothesized that a fat emulsion comprised of omega-3 fatty acids (i.e., fish oil), such as Omegaven,® would be beneficial in the management of steatotic liver injuiry by its inhibition of de novo lipogenesis, the reduction of arachidonic acid-derived inflammatory mediators, prevention of essential fatty acid deficiency through the presence of small amounts of arachidonic acid, and improved clearance of lipids from the serum. Animal studies have shown that IV fat emulsions such as fish oil that are high in eicosapentaenic and docosahexaenoic acid reduce impairment of bile flow which is seen in cholestasis caused by conventional fat emulsions. Furthermore, intravenous omega-3 fatty acids are well tolerated and might reduce the inflammatory effect in the liver of prolonged PN exposure and, potentially, reverse steatotic hepatic dysfunction. -

Short Bowel Syndrome with Intestinal Failure Were Randomized to Teduglutide (0.05 Mg/Kg/Day) Or Placebo for 24 Weeks
Short Bowel (Gut) Syndrome LaTasha Henry February 25th, 2016 Learning Objectives • Define SBS • Normal function of small bowel • Clinical Manifestation and Diagnosis • Management • Updates Basic Definition • A malabsorption disorder caused by the surgical removal of the small intestine, or rarely it is due to the complete dysfunction of a large segment of bowel. • Most cases are acquired, although some children are born with a congenital short bowel. Intestinal Failure • SBS is the most common cause of intestinal failure, the state in which an individual’s GI function is inadequate to maintain his/her nutrient and hydration status w/o intravenous or enteral supplementation. • In addition to SBS, diseases or congenital defects that cause severe malabsorption, bowel obstruction, and dysmotility (eg, pseudo- obstruction) are causes of intestinal failure. Causes of SBS • surgical resection for Crohn’s disease • Malignancy • Radiation • vascular insufficiency • necrotizing enterocolitis (pediatric) • congenital intestinal anomalies such as atresias or gastroschisis (pediatric) Length as a Determinant of Intestinal Function • The length of the small intestine is an important determinant of intestinal function • Infant normal length is approximately 125 cm at the start of the third trimester of gestation and 250 cm at term • <75 cm are at risk for SBS • Adult normal length is approximately 400 cm • Adults with residual small intestine of less than 180 cm are at risk for developing SBS; those with less than 60 cm of small intestine (but with a -

Total Parenteral Nutrition (TPN) in the Home Setting Corporate Medical Policy
Total Parenteral Nutrition (TPN) in the Home Setting Corporate Medical Policy File Name: Total Parenteral Nutrition (TPN) in the Home Setting File Code: UM.SPSVC.08 Origination: 10/2004 Last Review: 01/2020 Next Review: 01/2021 Effective Date: 04/01/2020 Description/Summary Total Parenteral Nutrition (TPN) is a type of infusion therapy that can be administered in the home setting, also known as parenteral hyper-alimentation. Used for patients with medical conditions that impair gastrointestinal absorption to a degree incompatible with life, is also used for variable periods of time to bolster the nutritional status of severely malnourished patients with medical or surgical conditions. TPN involves percutaneous transvenous implantation of a central venous catheter into the vena cava or right atrium. A nutritionally adequate hypertonic solution consisting of glucose (sugar), amino acids (protein), electrolytes (sodium, potassium), vitamins and minerals, and sometimes fats is administered daily. An infusion pump is generally used to assure a steady flow of the solution either on a continuous (24-hour) or intermittent schedule. If intermittent, a heparin lock device and diluted heparin are used to prevent clotting inside the catheter. Policy Coding Information Click the links below for attachments, coding tables & instructions. Attachment I- CPT® code list & instructions When a service may be considered medically Necessary Total Parenteral Nutrition (TPN) may be considered medically necessary by the Plan for conditions resulting in significantly -

Total Parenteral Nutrition (TPN)
FACT SHEET FOR PATIENTS AND FAMILIES Total Parenteral Nutrition (TPN) What is TPN? ExactaMix TPN Total Parenteral Nutrition (TPN) is a way to get carbohydrates, proteins, fats, vitamins, minerals, electrolytes, and water into your body through your veins. Preparing your TPN bag Drawing vitamins from a vial into a syringe: Remove the cap from vitamin bottles 1 and 2. (Each has a different colored cap.) Vigorously scrub the top of the vial with an 2 The patient or caregiver alcohol wipe. spikes the bag 3 The patient or with tubing. Using a 10 mL syringe, draw back 5 mL of air into caregiver injects the syringe. vitamins and other Do not use. medications into 1 The pharmacy Insert the needle into the vitamin bottle and inject the bag through uses this to the injection port. the 5 mL of air into the bottle. fill the bag. Hold the bottle upside down with the tip of the needle below the level of fluid. Draw 5 mL of vitamin bottle 1 into the syringe. Inject medications into the TPN bag Vigorously scrub the injection port on the TPN Withdraw the needle from bottle 1 and replace the bag with an alcohol wipe. needle cover. Inject vitamins and / or predrawn medication into Using a separate 10 mL syringe, repeat the process the injection port. with vitamin bottle 2. Withdraw the needle. Place the syringe and needle Using predrawn medications in a syringe: into a sharps container. Remove the cap from the end of syringe and discard Gently tilt the bag back and forth to mix. -

ESPEN Guidelines on Parenteral Nutrition: Gastroenterology
Clinical Nutrition 28 (2009) 415–427 Contents lists available at ScienceDirect Clinical Nutrition journal homepage: http://www.elsevier.com/locate/clnu ESPEN Guidelines on Parenteral Nutrition: Gastroenterology Andre´ Van Gossum a, Eduard Cabre b, Xavier He´buterne c, Palle Jeppesen d, Zeljko Krznaric e, Bernard Messing f, Jeremy Powell-Tuck g, Michael Staun d, Jeremy Nightingale h a Hoˆpital Erasme, Clinic of Intestinal Diseases and Nutrition Support, Brussels, Belgium b Hospital Universitari Germans Trias i Pujol, Department of Gastroenterology, Badalona, Spain c Hoˆpital L’Archet, Service de Gastro-ente´rologie et Nutrition, Nice, France d Rigshopitalet, Department of Gastroenterology, Copenhagen, Denmark e University Hospital Zagreb, Division of Gastroenterology and Clinical Nutrition, Zagreb, Croatia f Hoˆpital Beaujon, Service de Gastro-ente´rologie et Nutrition, Paris, France g The Royal London Hospital, Department of Human Nutrition, London, United Kingdom h St Mark’s Hospital, Department of Gastroenterology, Harrow, United Kingdom article info summary Article history: Undernutrition as well as specific nutrient deficiencies has been described in patients with Crohn’s Received 19 April 2009 disease (CD), ulcerative colitis (UC) and short bowel syndrome. In the latter, water and electrolytes Accepted 29 April 2009 disturbances may be a major problem. The present guidelines provide evidence-based recommendations for the indications, application and Keywords: type of parenteral formula to be used in acute and chronic phases of illness. Guidelines Parenteral nutrition is not recommended as a primary treatment in CD and UC. The use of parenteral Clinical practice nutrition is however reliable when oral/enteral feeding is not possible. Evidence-based Parenteral nutrition There is a lack of data supporting specific nutrients in these conditions. -

Human Health Toxicity Risk Assessment of DEHP in Medical Devices
1 Australian Government Department of Health and Ageing NICNAS Human Health Toxicity Risk Assessment of DEHP in Medical Devices NICNAS November 2007 334-336 llIawarra Rd, MARRICKVILLE NSW 2204 GPO Box 58 SYDNEY NSW 2001 Phone: 02 8577 8800 Fax: 02 8577 8888 WEBSITE: http://www.nicnas.gov.au 3 5. OVERSEAS AGENCY DECISIONS .................................................................................... 77 5.1 ·Health Canada ................................................................................................................ 77 5.2 US Food and Drug Administration ................ � .......... ..................................................... 79 5.3 European Commission ScientificCommittee on Medicinal Products and Medical Devices ....................................................................................................................................... 83 5.4 EU Risk Assessment (draft 2006) .................................... ...................................... ....... 84 5.5 NTP CERHR..................................... ............................................................................ 85 APPENDIX A: EXPOSURE CALCULATIONS ..................................................................... 87 ADULT ...................................................................................................................................... 87 NEONATES .............................................................................................................................. 98 APPEND IX B ............................................................................................................................ -

Medicare Coverage for Home Parenteral Nutrition – an Oxymoron? Part I
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #158 NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #158 Carol Rees Parrish, M.S., R.D., Series Editor Medicare Coverage for Home Parenteral Nutrition – An Oxymoron? Part I Penny Allen A 67 year old female patient with Stage III ovarian cancer presenting with partial small bowel obstruction, intractable nausea and vomiting is referred to a home infusion provider for home parenteral nutrition on a Friday afternoon. The patient has Medicare as her primary insurance as well as a supplemental policy. The physician and case manager are informed: “I am sorry, your patient does not meet the Medicare criteria for home parenteral nutrition.” INTRODUCTION hysicians and healthcare providers charged with and objective documentation still required by law today, caring for patients requiring home parenteral as well as strategies for attempting to provide home Pnutrition (HPN) face increasing pressure to infusion therapy when Medicare will not cover patients discharge patients earlier from the acute care setting. who appropriately need HPN. Regardless of insurance Patients with gastrointestinal (GI) disorders, GI and plan, if there is any possibility that a patient may require nutritional complications from cancer, and other HPN post discharge, the planning process should begin conditions may require continuation of parenteral immediately so that all members of the healthcare team nutrition (PN) therapy in the home setting. As the as well as the patient are aware of what is required to population of Medicare eligible beneficiaries grows, attempt to secure coverage. it is often a surprise at the time of discharge that many • What is needed to try to successfully qualify patients do not meet criteria for HPN and related this patient for HPN under Medicare? medically necessary infusion therapies under Medicare. -

Nutritional Management of Pediatric Short Bowel Syndrome
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #12 Series Editor: Carol Rees Parrish, M.S., R.D., CNSD Nutritional Management of Pediatric Short Bowel Syndrome Ana Abad-Sinden James Sutphen Pediatric short bowel syndrome (SBS), usually caused by massive intestinal resection, presents a significant nutritional challenge to the pediatric clinician. The overall clin- ical course and nutritional outcomes of SBS are impacted by various factors includ- ing remaining intestinal length and site, functional differences between the proximal and distal small intestine, and the presence of the colon. Nutritional management of SBS can be variable from patient to patient and can be divided into three stages: par- enteral nutrition, enteral nutrition, and introduction of solid foods. Optimization of parenteral nutrition with a balanced fuel mix of carbohydrate, protein and fat should be provided to meet energy needs and promote growth during the first few weeks of nutritional management. Following fluid and electrolyte stability and demonstrated growth on parenteral nutrition, enteral nutrition with semi-elemental or elemental formulas should be initiated in a timely manner to promote intestinal adaptation. Because electrolyte and fat-soluble vitamin loss can impair optimal growth, infants and children with SBS often require supplementation with sodium and pediatric mul- tivitamins and also benefit from supplementation with fiber and glutamine. Intro- duction of age appropriate low simple carbohydrate, high protein foods using small frequent feedings during the day further promote intestinal adaptation as well as oral-motor skills. The following article will present a comprehensive overview of nutritional management in the pediatric patient with SBS with practical guidelines for the clinician. -
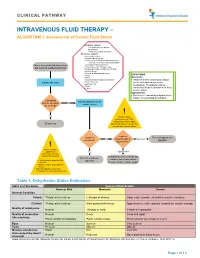
Intravenous Fluid Therapy – Algorithm 1
CLINICAL PATHWAY INTRAVENOUS FLUID THERAPY – ALGORITHM 1. Assessment of Overall Fluid Status Inclusion criteria: • All inpatients except those listed below • Patients pending admission Exclusion criteria: •Acute kidney injury •Chronic kidney disease •Endocrine or renal abnormalities leading to electrolyte derangements including DKA Patient who meets inclusion criteria •Oncology treatment protocol •Patients less than 30 days of age, and warrants supplemental fluids Including premature infants corrected for gestational age •Increased intracranial pressure •PICU DEFINITIONS •NICU Euvolemic: •Total Parenteral Nutrition dependent • Patient is at their ideal volume status Obtain vital signs •Pyloric Stenosis (neither dehydrated nor volume •Burn patients overloaded). The patient requires •Shock •Codes intravenous fluids to maintain their ideal volume status. Hypovolemic: • Patient is at least mildly dehydrated (see Table 1 for estimating dehydration) Can patient Assess patient’s current tolerate adequate No volume status enteral fluids? ! Yes Volume status assessment is 100% clinical. Do not rely upon Off pathway laboratory values to determine the patient’s volume status. Is patient Is patient Refer to hypovolemic No hypervolemic or Hypovolemic euvolemic? algorithm hypovolemic? ! Prior to starting a patient on Yes Hypervolemic maintenance IV fluids, consider the following: Reassess need for IV fluids and Refer to euvolemic consider issues with oncotic • Risk factors for abnormal ADH algorithm secretion pressure and/or cardiac output • Initial -

ESPEN Guidelines on Parenteral Nutrition: Central Venous Catheters (Access, Care, Diagnosis and Therapy of Complications)
Clinical Nutrition 28 (2009) 365–377 Contents lists available at ScienceDirect Clinical Nutrition journal homepage: http://www.elsevier.com/locate/clnu ESPEN Guidelines on Parenteral Nutrition: Central Venous Catheters (access, care, diagnosis and therapy of complications) Mauro Pittiruti a, Helen Hamilton b, Roberto Biffi c, John MacFie d, Marek Pertkiewicz e a Catholic University Hospital, Roma, Italy b John Radcliffe Infirmary, Oxford, United Kingdom c Division of Abdomino-Pelvic Surgery, European Institute of Oncology, Milano, Italy d Scarborough Hospital, Scarborough, United Kingdom e Medical University of Warsaw, Poland article info summary Article history: When planning parenteral nutrition (PN), the proper choice, insertion, and nursing of the venous access Received 4 February 2009 are of paramount importance. In hospitalized patients, PN can be delivered through short-term, non- Accepted 31 March 2009 tunneled central venous catheters, through peripherally inserted central catheters (PICC), or – for limited period of time and with limitation in the osmolarity and composition of the solution – through Keywords: peripheral venous access devices (short cannulas and midline catheters). Home PN usually requires PICCs Guidelines or – if planned for an extended or unlimited time – long-term venous access devices (tunneled catheters Evidence-based and totally implantable ports). Clinical practice Parenteral nutrition The most appropriate site for central venous access will take into account many factors, including the Central venous access patient’s conditions and the relative risk of infective and non-infective complications associated with Venous access devices each site. Ultrasound-guided venepuncture is strongly recommended for access to all central veins. For Midline catheters parenteral nutrition, the ideal position of the catheter tip is between the lower third of the superior cava PICC vein and the upper third of the right atrium; this should preferably be checked during the procedure. -

Parentral Nutrition Drug Compatibility.Pdf
PARENTERAL NUTRITION (PN) AND DRUG COMPATIBILITY PARENTERAL NUTRITION (PN) AND DRUG COMPATIBILITY The co-infusion of drugs and PN should be avoided. PN solutions are diverse in their The co-infusion of drugs and PN should be avoided. PN solutions are diverse in their composition and compatibilities with drugs can never be guaranteed. Drugs administered composition and compatibilities with drugs can never be guaranteed. Drugs administered to patients receiving PN should be given through a separate IV site or catheter lumen. If to patients receiving PN should be given through a separate IV site or catheter lumen. If a separate site is not available, the drug may be given through a separate line that has a separate site is not available, the drug may be given through a separate line that has a Y-connection to the PN line as close to the patient as possible. The PN should not a Y-connection to the PN line as close to the patient as possible. The PN should not be running and the common tubing must be adequately flushed before and after drug be running and the common tubing must be adequately flushed before and after drug administration. administration. Only if a patient’s clinical status requires uninterrupted PN administration can drugs Only if a patient’s clinical status requires uninterrupted PN administration can drugs on the following list be administered through the same Y-connection with the PN (amino on the following list be administered through the same Y-connection with the PN (amino acid/dextrose) still running. Note that drugs in the list have not been tested with lipids acid/dextrose) still running. -

ADVERSE EVENTS and UMBILICAL ARTERY INFUSIONS 1 The
Running head: ADVERSE EVENTS AND UMBILICAL ARTERY INFUSIONS 1 The Relationship Between Adverse Events and the Administration of Fluids, Medications, and Blood Products Through an Umbilical Arterial Catheter in Neonates Stacy M. Wallin, Lori Baas Rubarth, Carla Christensen, and Valerie Bibow Creighton University May 7, 2014 ADVERSE EVENTS AND UMBILICAL ARTERY INFUSIONS 2 Abstract The field of neonatology lacks adequate research to support or oppose the use of umbilical arterial catheters (UACs) for the administration of most fluids, medications, and blood products. There are recognized risks of having a UAC in place. However, it is unknown whether or not these risks increase with specific infusions. The purpose of this research was to identify and explain any relationships between adverse events and the infusion of fluids, medications, and blood products through UACs. The data collection process included a retrospective chart review of 104 infants in a 12‐bed level two neonatal intensive care unit who had a UAC placed. Medical records were examined for the number of indwelling catheter days, the size and position of the catheter, the composition and rate of infusates, and any complications possibly related to UAC use. Researchers examined the significance of any associations between adverse events and infusates and evaluated those relationships for practical significance. Relationships that could not be attributed to additional factors included: packed red blood cell transfusions and secondary port occlusion (p= .0259), furosemide and hypertension (p= .0108), and caffeine and hypertension (p= .0367). Further investigation into the safety of specific infusates is warranted. However, based on the results of this study the authors concluded that adverse outcomes are often not the result of infusions through the line as much as the presence of the UAC itself, and it may not be necessary to completely avoid UAC infusions.