Fifty Shades of Red: Lost Or Threatened Bryophytes in Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lejeuneaceae, Marchantiophyta)
Polish Botanical Journal 61(2): 205–229, 2016 e-ISSN 2084-4352 DOI: 10.1515/pbj-2016-0031 ISSN 1641-8190 CONTRIBUTION TO THE BRYOFLORA OF AUSTRALIA. VI. THE GENUS COLOLEJEUNEA (SPRUCE) STEPH. (LEJEUNEACEAE, MARCHANTIOPHYTA) Tamás Pócs Abstract. Thirty-eight species of the genus Cololejeunea (Spruce) Steph. (Lejeuneaceae, Marchantiophyta) are reported for the whole of Australia, more than doubling the number of taxa known since the first account of the Australian members of the genus. Cololejeunea cairnsiana Pócs, Cololejeunea heinari Pócs and Cololejeunea floccosa var. fraseriana Pócs are described as new to science. The records of Cololejeunea diaphana A. Evans, Cololejeunea gottschei (Steph.) Mizut., Cololejeunea longifolia (Mitt.) Benedix ex Mizut. Cololejeunea verrucosa Steph. and Cololejeunea floccosa(Lehm. & Lindenb.) Steph. var. amoenoides Tixier are new for the continent. Cololejeunea amoena Benedix is treated as a variety of Cololejeunea floccosa. Cololejeunea tortifolia Steph. is synonymized with Cololejeunea microscopica (Taylor) Schiffn. and is excluded from the Australian flora. The name Cololejeunea cambodiana Tixier is validated. A key for the Australian taxa and an analysis of their distribution follow the enumeration of species. Key words: Australia, Cololejeunea, distribution, epiphylls, liverworts, new species, taxonomy Tamás Pócs, Institute of Biology, Eszterházy Károly University Eger, Pf. 43, H-3301, Hungary; e-mail: [email protected] Introduction The first detailed account of the Australian rep- tory Program, during which I was accompanied resentatives of the genus Cololejeunea (Spruce) by local bryologists, among them Heinar Strei- Steph. was given by Thiers (1988), including mann, Andy Cairns, Elizabeth A. Brown, Robert 17 species, one among them new to science. Pócs G. -

Notes on Early Land Plants Today. 67. Notes on Lejeuneaceae Subtribus Cololejeuneinae (Marchantiophyta)
Phytotaxa 202 (1): 063–068 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.202.1.9 Notes on Early Land Plants Today. 67. Notes on Lejeuneaceae subtribus Cololejeuneinae (Marchantiophyta) TAMÁS PÓCS1, RUI-LIANG ZHU2, LARS SÖDERSTRÖM3, ANDERS HAGBORG4 & MATT VON KONRAT4 1Department of Botany, Eszterházy College, pf. 43, H-3301 Eger, Hungary; [email protected] 2Department of Biology, School of Life Sciences, East China Normal University, 3663 Zhong Shan North Road, Shanghai 200062, China; Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, East China Normal University, 500 Dongchuan Road, Shanghai 200241, China; [email protected] 3Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; [email protected] 5Department of Science and education, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605–2496, USA; hagborg@ pobox.com, [email protected] Abstract Cololejeunea subg. Aphanolejeunea is validated. Two new combinations, one validated species name, 14 new synonyms and four new lectotypes in Cololejeunea are proposed. The genus Austrolejeunea is shown to be invalid and all species are transferred to Nephelolejeunea making three new combinations and validating five species names. Introduction During the work with the forthcoming world checklist of liverworts and hornworts (Söderström et al., in press), several necessary taxonomic and nomenclature changes have come to our attention. Here we present necessary changes in Lejeuneaceae subtrib. Cololejeuneinae (sensu Gradstein 2013). In a molecular study Yu et al. (2014) showed the infrageneric structure in the genus Cololejeunea (Spruce 1884: 291) Stephani (1891: 208), but it does not correspond to the morphological structure currently used. -

Floristic Study of Bryophytes in a Subtropical Forest of Nabeup-Ri at Aewol Gotjawal, Jejudo Island
− pISSN 1225-8318 Korean J. Pl. Taxon. 48(1): 100 108 (2018) eISSN 2466-1546 https://doi.org/10.11110/kjpt.2018.48.1.100 Korean Journal of ORIGINAL ARTICLE Plant Taxonomy Floristic study of bryophytes in a subtropical forest of Nabeup-ri at Aewol Gotjawal, Jejudo Island Eun-Young YIM* and Hwa-Ja HYUN Warm Temperate and Subtropical Forest Research Center, National Institute of Forest Science, Seogwipo 63582, Korea (Received 24 February 2018; Revised 26 March 2018; Accepted 29 March 2018) ABSTRACT: This study presents a survey of bryophytes in a subtropical forest of Nabeup-ri, known as Geumsan Park, located at Aewol Gotjawal in the northwestern part of Jejudo Island, Korea. A total of 63 taxa belonging to Bryophyta (22 families 37 genera 44 species), Marchantiophyta (7 families 11 genera 18 species), and Antho- cerotophyta (1 family 1 genus 1 species) were determined, and the liverwort index was 30.2%. The predominant life form was the mat form. The rates of bryophytes dominating in mesic to hygric sites were higher than the bryophytes mainly observed in xeric habitats. These values indicate that such forests are widespread in this study area. Moreover, the rock was the substrate type, which plays a major role in providing micro-habitats for bryophytes. We suggest that more detailed studies of the bryophyte flora should be conducted on a regional scale to provide basic data for selecting indicator species of Gotjawal and evergreen broad-leaved forests on Jejudo Island. Keywords: bryophyte, Aewol Gotjawal, liverwort index, life-form Jejudo Island was formed by volcanic activities and has geological, ecological, and cultural aspects (Jeong et al., 2013; unique topological and geological features. -

Volatile Concentrate from the Neotropical Moss Neckeropsis Undulata (Hedw.) Reichardt, Existing in the Brazilian Amazon Thyago G
Miranda et al. BMC Chemistry (2021) 15:7 https://doi.org/10.1186/s13065-021-00736-3 BMC Chemistry RESEARCH ARTICLE Open Access Volatile concentrate from the neotropical moss Neckeropsis undulata (Hedw.) Reichardt, existing in the brazilian Amazon Thyago G. Miranda1, Raynon Joel M. Alves1, Ronilson F. de Souza2, José Guilherme S. Maia3, Pablo Luis B. Figueiredo2* and Ana Cláudia C. Tavares‑Martins1,2 Abstract Background: Many natural compounds have been identifed and synthesized by the advancement of bryophytes phytochemistry studies. This work aimed to report the composition of Neckeropsis undulata (Hedw.) Reichardt moss volatiles, sampled in the Combú Island, Belém city, Pará state, Brazil. The volatile concentrate of N. undulata was obtained by a simultaneous distillation‑extraction micro‑system, analyzed by GC and GC‑MS, and reported for the frst time. Results: Ten compounds were identifed in the volatile concentrate, corresponding to 91.6% of the total, being 1‑octen‑3‑ol (35.7%), α‑muurolol (21.4%), naphthalene (11.3%), and n‑hexanal (10.0 %) the main constituents. Most of the constituents of the N. undulata volatile concentrate have been previously identifed in other mosses, and liver‑ worts spread wide in the world. Conclusions: 1‑Octen‑3‑ol, n‑hexanal, 2‑ethylhexanol, isoamyl propionate, and octan‑3‑one are already known metabolic products obtained from enzymatic oxidation of polyunsaturated fatty acids, belonging to the large family of minor oxygenated compounds known as oxylipins. The knowledge of the composition of volatiles from moss N. undulata could contribute to the Neckeraceae species’ chemotaxonomy. Keywords: Neckeraceae, Volatile concentrate, 1‑octen‑3‑ol, α‑muurolol, n‑hexanal Background Brazil’s bryophyte fora comprises 1524 species, of which Bryophytes are small terrestrial spore-forming green 880 are mosses, 633 liverworts, and 11 hornworts. -

NEW DATA ABOUT MOSSES on the SVALBARD GLACIERS Olga
NEW DATA ABOUT MOSSES ON THE SVALBARD GLACIERS Olga Belkina Polar-Alpine Botanical Garden-Institute, Kola Science Center of the Russian Academy of Sciences, Apatity, Murmansk Province, Russia; e-mail: [email protected] Rapid melting and retreat of glaciers in the Arctic is a cause of sustainable long‐ In Svalbard moss populations were found on 9 glaciers. In 2012, during re‐examination of the populations a few term existence of ablation zone on them. Sometimes these areas are the habitats In 2007 B.R.Mavlyudov collected one specimen on individuals of Bryum cryophilum Mårtensson and some plants of some mosses partly due to good availability of water and cryoconite Bertilbreen (Paludella squarrosa (Hedw.) Brid.) and of Sanionia uncinata were found among H. polare shoots in substratum. 14 species were found in this unusual habitat on Alaska and Iceland: some specimens on Austre Grønfjordbreen (Ceratodon some large cushions. Therefore the next stage of cushion Andreaea rupestris Hedw., Ceratodon purpureus (Hedw.) Brid., Ditrichum purpureus (Hedw.) Brid., Warnstorfia sarmentosa succession had begun –emergence of a di‐ and multi‐species flexicaule (Schwaegr.) Hampe, Pohlia nutans (Hedw.) Lindb., Polytrichum (Wahlenb.) Hedenäs, Sanionia uncinata (Hedw.) community. A similar process was observed earlier on the juniperinum Hedw. (Benninghoff, 1955), Racomitrium fasciculare (Hedw.) Brid. Loeske, Hygrohypnella polare (Lindb.) Ignatov & bone of a mammal that was lying on the same glacier. (=Codriophorus fascicularis (Hedw.) Bendarek‐Ochyra et Ochyra) (Shacklette, Ignatova.). Ceratodon purpureus settled in center of almost spherical 1966), Drepanocladus berggrenii (C.Jens.) Broth. (Heusser, 1972), Racomitrium In 2009 populations of two latter species were studied cushion of Sanionia uncinata on the both butt‐ends of the crispulum var. -

Phylogeny and Classification of Lejeuneaceae Subtribe Cheilolejeuneinae (Marchantiophyta) Based on Nuclear and Plastid Molecular Markers
Cryptogamie, Bryologie, 2015, 36 (4): 313-333 © 2015 Adac. Tous droits réservés Phylogeny and classification of Lejeuneaceae subtribe Cheilolejeuneinae (Marchantiophyta) based on nuclear and plastid molecular markers Wen YE a,b, S. Robbert GRADSTEIN c, A. Jonathan SHAW d, Blanka SHAW d, Boon-Chuan HO e, Alfons SCHÄFER-VErwIMP f, Tamás PÓCS g, Jochen HEINRICHS h & Rui-Liang ZHU b* aKey Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Xingke Road 723, Tianhe District, Guangzhou 510650, P. R. China bDepartment of Biology, School of Life Sciences, East China Normal University, 3663 Zhong Shan North Road, Shanghai 200062, P. R. China cMuséum National d’Histoire Naturelle, Dept. Systématique et Evolution, Case Postale 39, 57 rue Cuvier, 75231 Paris cedex 05, France dBiology Department, Duke University, Box 90338 Durham, NC 27708, USA eSingapore Botanic Gardens, 1 Cluny Road, Singapore 259569, Republic of Singapore fMittlere Letten 11, 88634 Herdwangen-Schönach, Germany gBiology Institute of Eszterházy College, Eger, Pf. 43, H-3301, Hungary hDepartment of Biology I, University of Munich (LMU), Systematic Botany and Mycology, GeoBio-Center, Menzinger Strasse 67, 80638 Munich, Germany Abstract – Cheilolejeuneinae is an early diverging lineage of Lejeuneaceae tribe Lejeuneeae with a pantropical distribution. The current phylogeny and classification of this subtribe is based on morphological and limited-sampling molecular studies. Here we present a molecular phylogeny of Cheilolejeuneinae and related lineages based on maximum parsimony and maximum likelihood analyses, as well as Bayesian inference of two chloroplast regions (trnL-F, trnG) and the nuclear ribosomal ITS1-5.8S-ITS2 region, to test the monophyly of this subtribe, and to re-evaluate the infrageneric classification of Cheilolejeunea. -

Fifty Shades of Red: Lost Or Threatened Bryophytes in Africa
Bothalia - African Biodiversity & Conservation ISSN: (Online) 2311-9284, (Print) 0006-8241 Page 1 of 7 Original Research Fifty shades of red: Lost or threatened bryophytes in Africa Authors: Background: A Red List of threatened bryophytes is lacking for Africa. The International 1,2 Jacques van Rooy Union for Conservation of Nature (IUCN) Species Survival Commission (SSC) Bryophyte Ariel Bergamini3 Irene Bisang4 Specialist Group has recently launched the ‘Top 10 Initiative’ to identify the 10 species on each continent that are at highest risk of extinction. Affiliations: 1National Herbarium, South Objectives: The main aim of this paper was to highlight some of the lost or strongly threatened African National Biodiversity bryophyte species in sub-Saharan Africa and the East African islands and to draw up a Top 10 Institute, South Africa list for Africa. 2School of Animal, Method: Lost or threatened species have been identified with the help of experts on the Plant and Environmental bryoflora of Africa, global and regional Red Lists and taxonomic literature. Each species on Sciences, University of the this candidate list is discussed at the hand of its taxonomy, distribution, habitat, threat and Witwatersrand, South Africa current global or regional Red List status as far as previously assessed. 3 Department of Biodiversity Results: Fifty bryophyte species, representing 40 genera and 23 families, have been identified and Conservation Biology, Swiss Federal Research as Top 10 candidates. Of these, 29 are endemic to Africa and 21 are restricted to the East African Institute WSL, Switzerland islands. The majority of the candidate species occur in one of eight ‘biodiversity hotspots’ with most species (19) in the Madagascar and the Indian Ocean Islands hotspot. -

Total of 10 Pages Only May Be Xeroxed
CENTRE FOR NEWFOUNDLAND STUDIES TOTAL OF 10 PAGES ONLY MAY BE XEROXED (Without Author's Permission) ,, l • ...J ..... The Disjunct Bryophyte Element of the Gulf of St. Lawrence Region: Glacial and Postglacial Dispersal and Migrational Histories By @Rene J. Belland B.Sc., M.Sc. A thesis submitted to the School of Graduate Studies in partial fulfilment of the requirements for the degree of Doctor of Philosophy Department of Biology Memorial University of Newfoundland December, 1Q84 St. John's Newfoundland Abstract The Gulf St. Lawrence region has a bryophyte flora of 698 species. Of these 267 (38%) are disjunct to this region from western North America, eastern Asia, or Europe. The Gulf of St. Lawrence and eastern North American distributions of the disjuncts were analysed and their possible migrational and dispersal histories during and after the Last Glaciation (Wisconsin) examined. Based on eastern North American distribution patterns, the disjuncts fell into 22 sub elements supporting five migrational/ dispersal histories or combinations of these : (1) migration from the south, (2) migration from the north, (3) migration from the west, (4) survival in refugia, and (5) introduction by man. The largest groups of disjuncts had eastern North American distributions supporting either survival of bryophytes in Wisconsin ice-free areas of the Gulf of St. Lawrence or postglacial migration to the Gulf from the south. About 26% of the disjuncts have complex histories and their distributions support two histories. These may have migrated to the Gulf from the west and/or north, or from the west and/or survived glaciation in Gulf ice-free areas. -

Article ISSN 1179-3163 (Online Edition)
Phytotaxa 63: 21–68 (2012) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2012 Magnolia Press Article ISSN 1179-3163 (online edition) Early Land Plants Today: Index of Liverworts & Hornworts 2009–2010 LARS SÖDERSTRÖM1, ANDERS HAGBORG2, MARSHALL R. CROSBY3 & MATT VON KONRAT2 1 Department of Biology, Norwegian University of Science and Technology, N-7491, Trondheim, Norway; [email protected] 2 Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605–2496, U.S.A.;[email protected], [email protected] 3 Missouri Botanical Garden, P. O. Box 299, St. Louis, MO 63166–0299 U.S.A.; [email protected] Abstract A widely accessible list of known plant species is a fundamental requirement for plant conservation and has vast applications. An index of published names of liverworts and hornworts between 2009 and 2010 is provided as part of a continued effort in working toward producing a world checklist of this group. Included in the list are also names overlooked by earlier indices. The list includes 30 higher taxa, 250 species, 52 infraspecific taxa, 31 autonyms, and two fossils for 2009 and 2010. A number of taxa not covered by the earlier indices for 2000-2008 are also included. Key words: Liverworts, hornworts, index, nomenclature Introduction Under the auspices of the Early Land Plants Today project, there has been a strong community-driven effort attempting to address the critical need to synthesize the vast nomenclatural, taxonomic and global distributional data for liverworts (Marchantiophyta) and hornworts (Anthocerotophyta) (von Konrat et al. 2010a). These endeavours are critical in providing the foundation to develop a working checklist of liverworts and hornworts worldwide; the first version is projected to be published in 2012. -
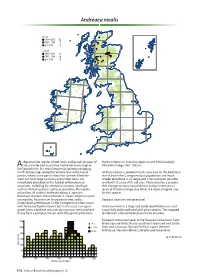
Andreaea Nivalis
Andreaea nivalis Britain 1990–2013 12 1950–1989 7 pre-1950 4 Ireland 1990–2013 0 1950–1989 0 pre-1950 0 characteristic species of wet rocks and gravels in areas of Nardia compressa, Scapania uliginosa and Pohlia ludwigii. Alate snow-lie and associated meltwater burns high in Altitudinal range: 880–1340 m. the Scottish hills. It is most frequently found on dripping, north-facing crags along the ‘cornice line’ at the top of Andreaea nivalis is abundant in its core area on the Ben Nevis corries, where snow persists into the summer. Here the massif and in the Cairngorms but populations are much moss can form large cushions and on Ben Nevis it is smaller elsewhere in its range and it has not been possible remarkably abundant in this habitat with numerous to refind it at some of its old sites. There must be a concern associates, including Deschampsia cespitosa, Saxifraga that changes in snow accumulation and persistence as a stellaris, Anthelia julacea, Lophozia opacifolia, Marsupella result of climate change may affect the more marginal sites sphacelata, M. stableri, Andreaea alpina, A. rupestris, for this species. Ditrichum zonatum, Kiaeria falcata, K. starkei, Polytrichastrum sexangulare, Racomitrium lanuginosum and, rarely, Dioicous; capsules are occasional. Oedipodium griffithianum. In the Cairngorms it often occurs with Andreaea frigida in burns but it also occurs on open Andreaea nivalis is a large and easily identifiable moss and gravel areas which are very wet during snow melt and here is not likely to be confused with other species. The mapped it may form a complex mosaic with Marsupella sphacelata, distribution is therefore believed to be accurate. -

New Species of Pelargonium (Geraniaceae) from Namaqualand
50 S. Afr. J. [Jot. 1999. 65(1): 50-58 New species of Pelargonium (Geraniaceae) from Namaqualand Elizabeth M. Marais Department of Bolany, University of Stellenbosch. Private Bag X1 , Matieland, 7602 Republic of South Africa e~ma i l: [email protected] Received 27 July 1998; revised 5 October 1998 Pelargonium angustipelalum E.M. Marais, P. parvipetalum E.M . Marais and P. rubiginosum E.M Marais are described as new species. Although all three of them are tuberous species with turnip-shaped lubers covered with dark brown peeling periderms and apically a short flattened stem from which the leaves and scape emerge, and thus belonging to section Hoarea (Sweet) DC. , they have different types of floral structures. To ascertain their interrelationships within section Hoarea, their macromorphological characters, leaf anatomy and pollen morphology are compared to those of other species within section Hoarea. Illustrations of the three species as well as electronmicrographs of their pollen grains and a distribution map are provided. Keywords: Geraniaceae, Hoarea, new species, Pelargonium, taxonomy. Introduction systematic position of these three species within section Hoarea, J)eJargolliu11I angllslipelalu11I E.M. Marais, P. pnrvipelaJum E.M. their macro morphological characters, leaf anatomy and pollen Marais and P. rllbiginosum E.M. Marais are deciduous geo morphology were compared to those of other species within phytes with regularly or turnip-shaped tubers covered with dark secti on Hoarea. brown peeling periderms and apically a short flattened stem from which the leaves and scape emerge, and thus belonging to the Materials and Methods section Hoarea (Sweet) DC. All three species occur in Morphological studies were pl!rformed on herbarium specimens and Namaqualand in areas with an annual rainfall of less than 200 on plants coiIected in the field and cultivated in the Botanical Gar mm. -

Species Richness and Composition of Epiphytic Bryophytes in Flooded Forests of Caxiuanã National Forest, Eastern Amazon, Brazil
Anais da Academia Brasileira de Ciências (2017) 89(3 Suppl.): 2371-2382 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201720160860 www.scielo.br/aabc | www.fb.com/aabcjournal Species richness and composition of epiphytic bryophytes in flooded forests of Caxiuanã National Forest, Eastern Amazon, Brazil GABRIELA R. CERQUEIRA1, ANNA LUIZA ILKIU-BORGES1 and LEANDRO V. FERREIRA2 1Programa de Pós-Graduação em Ciências Biológicas - Botânica Tropical, Universidade Federal Rural da Amazônia e Museu Paraense Emílio Goeldi, Av. Perimetral, 1901, 66077-530 Belém, PA, Brazil 2Museu Paraense Emílio Goeldi, Av. Perimetral, 1901, 66077-530 Belém, PA, Brazil Manuscript received on December 12, 2016; accepted for publication on June 29, 2017 ABSTRACT This study aimed to compare the richness and composition of the epiphytic bryoflora between várzea and igapó forests in Caxiuanã National Forest, Brazilian Amazon. Bryophytes were collected on 502 phorophytes of Virola surinamensis. Average richness per phorophyte and composition between forests and between dry and rainy periods was tested by two-way analysis and by cluster analysis, respectively. In total, 54 species of 13 families were identified. Richness was greater inigapó forest (44 species) compared to várzea forest (38 species). There was no significant difference in the number of species between the studied periods. Cluster analysis showed the bryoflora composition was different between várzea and igapó, but not between dry and rainy periods. Results did not corroborate the hypothesis that várzea forests harbor higher species richness than igapó forests. Key words: Floristics, liverworts, mosses, seasonality.