Open Source Drug Discovery- a New Paradigm of Collaborative Research in Tuberculosis Drug Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2017-2018
ANNUAL REPORT IISc 2017-18 INDIAN INSTITUTE OF SCIENCE VISITOR The President of India PRESIDENT OF THE COURT N Chandrasekaran CHAIRMAN OF THE COUNCIL P Rama Rao DIRECTOR Anurag Kumar DEANS SCIENCE: Biman Bagchi ENGINEERING: K Kesava Rao UG PROGRAMME: Anjali A Karande REGISTRAR V Rajarajan Pg 3 IISc RANKED INDIA’S TOP UNIVERSITY In 2016, IISc was ranked Number 1 among universities by the National Institutional Ranking Framework (NIRF) under the auspices of the Ministry of Human Resource Development. It was the first time the NIRF came out with rankings for Indian universities and institutions of higher education. In both 2017 and 2018, the Institute was again ranked first among universities, as well as first in the overall category. CONTENTS Foreword IISc at a Glance 8 1. The Institute 18 Court 5 Council 20 Finance Committee 21 Senate 21 Faculties 21 2. Staff (administration) 22 3. Divisions 25 3.1 Biological Sciences 26 3.2 Chemical Sciences 58 3.3 Electrical, Electronics, and Computer Sciences 86 3.4 Interdisciplinary Research 110 3.5 Mechanical Sciences 140 3.6 Physical and Mathematical Science 180 3.7 Centres under the Director 206 4. Undergraduate Programme 252 5. Awards/Distinctions 254 6. Students 266 6.1 Admissions & On Roll 267 6.2 SC/ST Students 267 6.3 Scholarships/Fellowships 267 6.4 Assistance Programme 267 6.5 Students Council 267 6.6 Hostels 267 6.7 Institute Medals 268 6.8 Awards & Distinctions 269 6.9 Placement 279 6.10 External Registration Program 279 6.11 Research Conferments 280 7. Events 300 7.1 Institute Lectures 310 7.2 Conferences/Seminars/Symposia/Workshops 302 8. -

List of Life Members As on 20Th January 2021
LIST OF LIFE MEMBERS AS ON 20TH JANUARY 2021 10. Dr. SAURABH CHANDRA SAXENA(2154) ALIGARH S/O NAGESH CHANDRA SAXENA POST HARDNAGANJ 1. Dr. SAAD TAYYAB DIST ALIGARH 202 125 UP INTERDISCIPLINARY BIOTECHNOLOGY [email protected] UNIT, ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 11. Dr. SHAGUFTA MOIN (1261) [email protected] DEPT. OF BIOCHEMISTRY J. N. MEDICAL COLLEGE 2. Dr. HAMMAD AHMAD SHADAB G. G.(1454) ALIGARH MUSLIM UNIVERSITY 31 SECTOR OF GENETICS ALIGARH 202 002 DEPT. OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY 12. SHAIK NISAR ALI (3769) ALIGARH 202 002 DEPT. OF BIOCHEMISTRY FACULTY OF LIFE SCIENCE 3. Dr. INDU SAXENA (1838) ALIGARH MUSLIM UNIVERSITY, ALIGARH 202 002 HIG 30, ADA COLONY [email protected] AVANTEKA PHASE I RAMGHAT ROAD, ALIGARH 202 001 13. DR. MAHAMMAD REHAN AJMAL KHAN (4157) 4/570, Z-5, NOOR MANZIL COMPOUND 4. Dr. (MRS) KHUSHTAR ANWAR SALMAN(3332) DIDHPUR, CIVIL LINES DEPT. OF BIOCHEMISTRY ALIGARH UP 202 002 JAWAHARLAL NEHRU MEDICAL COLLEGE [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 14. DR. HINA YOUNUS (4281) [email protected] INTERDISCIPLINARY BIOTECHNOLOGY UNIT ALIGARH MUSLIM UNIVERSITY 5. Dr. MOHAMMAD TABISH (2226) ALIGARH U.P. 202 002 DEPT. OF BIOCHEMISTRY [email protected] FACULTY OF LIFE SCIENCES ALIGARH MUSLIM UNIVERSITY 15. DR. IMTIYAZ YOUSUF (4355) ALIGARH 202 002 DEPT OF CHEMISTRY, [email protected] ALIGARH MUSLIM UNIVERSITY, ALIGARH, UP 202002 6. Dr. MOHAMMAD AFZAL (1101) [email protected] DEPT. OF ZOOLOGY [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 ALLAHABAD 7. Dr. RIAZ AHMAD(1754) SECTION OF GENETICS 16. -

Presentation on the Division of Biological
INDIAN INSTITUTE OF SCIENCE (IISc) Bangalore IISc: THE DIVISIONAL STRUCTURE Biological Chemical Sciences Sciences Physical and Interdisciplinary Mathematical Sciences IISc Research Mechanical Electrical Sciences Sciences Division of Biological Sciences (76 Serving faculty members) (>250 publications/year) Molecular Biochemistry Microbiology and Cell Biology Biophysics Unit (1921) (1941) (1971) Prof. C. Jayabaskaran Prof. U. Vijayraghavan Prof. N. Srinivasan Centre for Molecular Reproduction, Centre for Development and Ecological Sciences Genetics Neuroscience (1983) (1989) (2009) Prof. R. Balakrishnan Prof. S. Visweswariah Prof. A. Murthy ~60 PhDs Central Animal Centre for Infectious graduate Facility Disease Research each year (1971) (2014) Prof. K. Somasundaram Prof. D. Nandi Other Centres for Interdisciplinary Research 1. Centre for Biosystems Science and Engineering (BSSE) 2. Centre for Nano Science and Engineering (CeNSE) 3. National Mathematics Initiative (IISc node; Math- Bio@IISc) 4. Centre for Brain Research (CBR) Funding generated by the Departments/Centres in the Division of Biological Sciences (2014-18) Total: INR ~402 crores (US$~57 million) (Excludes IISc and DBT-IISc Funds) CAF CIDR BC MRDG CES MBU CNS DBT-IISc Funds MCB 2012-2018: ~70 crores 2019-2021: ~78 crores Funding sources Indo-Swedish grant Indo-Australia grant Indo-Israel grant Indo-Russian grant 1. Disease Biology 1.1 Infectious disease biology (Viruses, bacteria and fungi) Principal Investigators from DBS: Dipshikha Chakravortty, S. Vijaya, K. N. Balaji, P. Ajitkumar, Amit Singh, S. Mahadevan, N. Ganesh, Dipankar Nandi, Saibal Chatterjee, and Utpal Tatu. Co-investigators from other divisions: Jagadeesh Gopalan (Aerospace Engineering), Ashok Raichur (Materials Engineering), G. Mugesh (Inorganic Physical Chemistry), and S. Umapathy (Inorganic Physical Chemistry). 1. -

Awardees of National Bioscience Award for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARD FOR CAREER DEVELOPMENT Awardees for the year 2016 1. Dr. Mukesh Jain, Associate Professor, School of Computational and Integrative Sciences, Jawaharlal Nehru University, New Delhi-110067 2. Dr. Samir K. Maji, Associate Professor, Indian Institute of Technology, Powai, Mumbai- 400076 3. Dr. Anindita Ukil, Assistant Professor, Calcutta University, Kolkata 4. Dr. Arnab Mukhopadhyay, Staff Scientist V, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi- 110067 5. Dr. Rohit Srivastava, Professor, Indian Institute of Technology, Bombay, Mumbai- 400076 6. Dr. Pinaki Talukdar, Associate Professor, Indian Institute of Science Education and Research, Dr. Homi Bhabha Road, Pashan, Pune- 7. Dr. Rajnish Kumar Chaturvedi, Senior Scientist, CSIR- Indian Institute of Toxicology Research, Lucknow-226001 8. Dr. Jackson James, Scientist E-II, Neuro Stem Cell Biology Lab, Neurobiology Division, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala- 695014 Awardees for the year 2015 1. Dr. Sanjeev Das, Staff Scientist-V, National Institute of Immunology, New Delhi 2. Dr. Ganesh Nagaraju, Assistant Professor, Department of Biotechnology, Indian Institute of Science, Bangalore- 5600012. 3. Dr. Suvendra Nath Bhattacharya, Principal Scientist, CSIR- Indian Institute of Chemical Biology, Kolkata- 700032 4. Dr. Thulasiram H V, Principal Scientist, CSIR-National Chemical Laboratory, Pune- 411008. 5. Dr. Pawan Gupta, Principal Scientist, Institute of microbial Technology, Chandigarh- 160036. 6. Dr. Souvik Maiti, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025. 7. Dr. Pravindra Kumar, Associate Professor, Department of Biotechnology, IIT, Roorkee- 247667. 8. Dr. Anurag Agrawal, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025 9. Dr. Gridhar Kumar Pandey, Professor, Department of Plant Molecular Biology, University of Delhi South Campus, New Delhi- 110067 10. -

National Bioscience Awards for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARDS FOR CAREER DEVELOPMENT Awardees for the year 2012 1. Dr. Kaustuv Sanyal, Associate Professor, Molecular Mycology Laboratory, Molecular Biology & Genetics Unit, Jawaharlal Nehru Centre for Advance Scientific Research, Jakkur P.O. Bangalore 560064 2. Dr Naval Kishore Vikram, Associate Professor, Department of Medicine, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, New Delhi- 110029 3. Dr. Aditya Bhushan Pant, Senior Scientist & In-charge, In Vitro Toxicology Laboratory, Indian Institute of Toxicology Research, PO Box: 80, MG Marg, Lucknow 226001 (UP) India 4. Dr. Subrata Adak, Senior Scientist, Indian Institute of Chemical Biology; 4, Raja S.C. Mullick Road, Kolkata-700032 5. Dr. Durai Sundar, Assistant Professor, Dept of Biochemical Engineering & Biotechnology, Indian Institute of Technology (IIT) Delhi, Hauz Khas, New Delhi – 110016 6. Dr S Venkata Mohan, Senior Scientist, Bioengineering and Environmental Centre (BEEC) CSIR-Indian Institute of Chemical Technology, Hyderabad-500 607 7. Dr. Munia Ganguli, Scientist E-I, CSIR-Institute of Genomics & Integrative Biology, Mall Road,New Delhi 110 007 8. Dr. Asad U Khan, Associate Professor & Coordinator/Head of Biotechnology Department, A.M.U, Interdisciplinary Biotechnology Unit, A.M.U., Aligarh 202002 9. Dr. Sathees C. Raghavan, Assistant Professor, Department of Biochemistry, Indian Institute of Science, Bangalore 560 012 10. Dr. Vidita A. Vaidya, Associate Professor, Department of Biological Sciences, Tata Institute of Fundamental Research, 1, Homi Bhabha Road, Colaba, Mumbai - 400005 Awardees for the year 2011 1. Dr. M. M. Parida, Scientist-F, Joint Director, Division of Virology Defence R & D Establishment, DRDE, DRDO, Ministry of Defence, Jhansi Road, Gwalior- 474002 2. -

Consultancy Brochure
Consultancy Brochure Centre for Scientific & Industrial Consultancy Indian Institute of Science Centre for Scientific and Industrial Consultancy Indian Institute of Science Bangalore The Indian Institute of Science (IISc) was founded in 1909 by the visionary industrialist, Jamsetji Nusserwanji Tata, ".. to provide for advanced instruction and to conduct original investigations in all branches of knowledge". He envisaged that the Institute should work ".. in particular, in such branches of knowledge as are likely to promote the material and industrial welfare of India". As IISc has been interacting with industries since its very inception through individual informal contacts of faculty members, this interaction was given an institutional backing with the establishment of the Centre for Scientific and Industrial Consultancy (CSIC) in 1975. The role of CSIC is to strengthen, promote and streamline the interaction between IISc and industry. The goal of CSIC is to promote indigenous technologies to make the nation self-reliant in all aspects, and to nurture fruitful relationships between faculty members of IISc and industries. CSIC views consultancy projects as technically sub-product development and structural analysis are challenging collaborative efforts between the Institute and carried out. the Industry/sponsor. The participation of both sides is adaptive and is often tuned to exploit the strengths and Diagnostics, Proof checking, Feasibility evaluations, complimentary expertise available. Services undertaken System studies by CSIC can be broadly grouped to In this mode of engagement, the consultant from IISc ➢ Design and development of products/ process/ evaluates an design proposal or helps in diagnosis of software/ systems problems in an existing construction/structure or a new ➢ Technical advice and guidance for design and suggests remedial measures. -

The Year Book 2020
THE YEAR BOOK 2020 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-23616094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2020 Indian Academy of Sciences Information in this Year Book is updated up to 31 January 2020. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by The Print Point, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: -

The Year Book 2021
THE YEAR BOOK 2021 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-2361 6094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2021 Indian Academy of Sciences Information in this Year Book is updated up to 15 February 2021. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Mobile: 97310 01283) Printed by Ekakshara Printers, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: Fellowship -
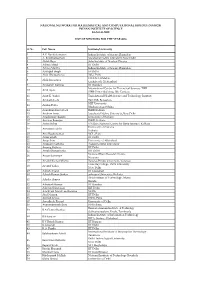
Sl No Full Name Institute/University 1 a K Nandakumaran Indian Institute
NATIONAL NETWORK FOR MATHEMATICAL AND COMPUTATIONAL BIOLOGY (NNMCB) INDIAN INSTITUTE OF SCIENCE BANGALORE LIST OF MENTORS FOR THE YEAR 2016 Sl No Full Name Institute/University 1 A K Nandakumaran Indian Institute of Science,Bangalore 2 A. Krishnamachari Jawaharlal Nehru University,New Delhi 3 Abhik Basu Saha Institute of Nuclear Physics 4 Aditya Mittal IIT Delhi 5 Aditya Murthy Indian Institute of Science,Bangalore 6 Ajit Iqbal Singh ISI Delhi 7 Alok Bhattacharya JNU, Delhi CR RAO AIMSCS 8 Alok Srivastava Gachibowli, Hyderabad 9 Ambarish Kunwar IIT Bombay International Centre for Theoretical Sciences-TIFR 10 Amit Apte TIFR Centre Building, IISc Campus 11 Amit K. Yadav Translational Health Science and Technology Institute 12 Amitabh Joshi JNCASR, Bangalore KIIT University 13 Amlan Datta Bhubaneswar,Odisha 14 Anandamohan Ghosh IISER Kolkata 15 Andrew Lynn Jawaharlal Nehru University,New Delhi 16 Angshuman Bagchi University of Kalyani 17 Anirban Banerjee IISER, Kolkata 18 Anita Mehta S N Bose National Centre for Basic Sciences, Kolkata University of Calcutta 19 Ansuman Lahiri Kolkata 20 Anu Raghunathan NCL, Pune 21 Anup Singh IIT Delhi 22 Anup Som University of Allahabad 23 Anupam Nath Jha Tezpur Central University 24 Anurag Rathore IIT Delhi 25 Arnab Bhattacherjee IIIT Delhi National Brain Research Centre 26 Arpan Bannerjee Haryana 27 Arvind Kumar Mishra Banaras Hindu University, Varanasi Hans Raj College, Delhi University 28 Arvind Yadav New Delhi 29 Ashish Anand IIT Guwahati 30 Ashish Kumar Sarkar jadavpur University, Kolkata Birla Institute of Technology, Mesra 31 Ashoke Sharon Ranchi 32 Ashutosh Kumar IIT Bombay 33 Ashwin Srinivasan IIIT Delhi 34 Aswin Sai Narain Seshasayee NCBS 35 Atul Narang IIT Delhi 36 Aurnab Ghose IISER, Pune 37 Awadhesh Prasad University of Delhi 38 Ayanendranath Basu ISI-Kolkata. -

List of Life Members As on 1St Frbruary 2018
LIST OF LIFE MEMBERS AS ON 1ST FRBRUARY 2018 ALIGARH 10. Dr. SHAGUFTA MOIN (1261) DEPT. OF BIOCHEMISTRY 1. Dr. HAMMAD AHMAD SHADAB G. G.(1454) J. N. MEDICAL COLLEGE 31 SECTOR OF GENETICS ALIGARH MUSLIM UNIVERSITY DEPT. OF ZOOLOGY ALIGARH 202 002 ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 11. SHAIK NISAR ALI (3769) DEPT. OF BIOCHEMISTRY 2. Dr. INDU SAXENA (1838) FACULTY OF LIFE SCIENCE HIG 30, ADA COLONY ALIGARH MUSLIM UNIVERSITY AVANTEKA PHASE I ALIGARH 202 002 RAMGHAT ROAD, ALIGARH 202 001 [email protected] 3. Dr. (MRS) KHUSHTAR ANWAR SALMAN(3332) ALLAHABAD DEPT. OF BIOCHEMISTRY JAWAHARLAL NEHRU MEDICAL COLLEGE 12. Dr. ABHAY KUMAR PANDEY (2054) ALIGARH MUSLIM UNIVERSITY DEPT OF BIOCHEMISTRY ALIGARH 202 002 UNIVERSITY OF ALLAHABAD [email protected] ALLAHABAD 211002 4. Dr. MOHAMMAD TABISH (2226) 13. Dr. AMIT KUMAR (2123) DEPT. OF BIOCHEMISTRY DEPT. OF ZOOLOGY FACULTY OF LIFE SCIENCES ALLAHABAD UNIVERSITY ALIGARH MUSLIM UNIVERSITY ALLAHABAD 211 002 ALIGARH 202 002 [email protected] 14. Dr. AMITA MISHRA (2665) C/O SRI RAMESH MISHRA 5. Dr. MOHAMMAD AFZAL (1101) G B 53, GANGA SECTOR, TRIVENIPURAM DEPT. OF ZOOLOGY JHUSI , ALLAHABAD 211 019 ALIGARH MUSLIM UNIVERSITY [email protected] ALIGARH 202 002 15. Dr. ANJANA PANDEY (1549) 6. Dr. RIAZ AHMAD(1754) CENTRE FOR BIOTECHNOLOGY SECTION OF GENETICS MOTILAL NEHRU NATIONAL INSTITUTE OF DEPT. OF ZOOLOGY TECHNOLOGY ALIGARH MUSLIM UNIVERSITY ALLAHABAD 211 004 ALIGARH 202 002 16. ASHUTOSH GUPTA (3789) 7. Dr. ROSHAN ALAM (1958) 116A/7B JAYANTIPUR C/O MOHD HABIB EDUCATIONAL HOUSE SULEM SARAI AMU SHAM SHAD MARKET ALLAHABAD 211 011 ALIGARH 202 002 [email protected] 8. -

List of Life Members As on 1St December 2018
LIST OF LIFE MEMBERS AS ON 1ST DECEMBER 2018 10. Dr. SHAGUFTA MOIN (1261) ALIGARH DEPT. OF BIOCHEMISTRY J. N. MEDICAL COLLEGE 1. Dr. HAMMAD AHMAD SHADAB G. G.(1454) ALIGARH MUSLIM UNIVERSITY 31 SECTOR OF GENETICS ALIGARH 202 002 DEPT. OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY 11. SHAIK NISAR ALI (3769) ALIGARH 202 002 DEPT. OF BIOCHEMISTRY FACULTY OF LIFE SCIENCE 2. Dr. INDU SAXENA (1838) ALIGARH MUSLIM UNIVERSITY HIG 30, ADA COLONY ALIGARH 202 002 AVANTEKA PHASE I [email protected] RAMGHAT ROAD, ALIGARH 202 001 12. DR. MAHAMMAD REHAN AJMAL KHAN (4157) 3. Dr. (MRS) KHUSHTAR ANWAR SALMAN(3332) 4/570, Z-5, NOOR MANZIL COMPOUND DEPT. OF BIOCHEMISTRY DIDHPUR, CIVIL LINES JAWAHARLAL NEHRU MEDICAL COLLEGE ALIGARH UP 202 002 ALIGARH MUSLIM UNIVERSITY [email protected] ALIGARH 202 002 [email protected] ALLAHABAD 4. Dr. MOHAMMAD TABISH (2226) DEPT. OF BIOCHEMISTRY 13. Dr. B. SHARMA (872) FACULTY OF LIFE SCIENCES DEPARTMENT OF BIOCHEMISTRY ALIGARH MUSLIM UNIVERSITY UNIVERSITY OF ALLAHABAD ALIGARH 202 002 ALLAHABAD 211002 INDIA [email protected] [email protected] [email protected] 5. Dr. MOHAMMAD AFZAL (1101) 9415715639 DEPT. OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY 14. Dr. ABHAY KUMAR PANDEY (2054) ALIGARH 202 002 DEPT OF BIOCHEMISTRY UNIVERSITY OF ALLAHABAD 6. Dr. RIAZ AHMAD(1754) ALLAHABAD 211002 SECTION OF GENETICS DEPT. OF ZOOLOGY 15. Dr. AMIT KUMAR (2123) ALIGARH MUSLIM UNIVERSITY DEPT. OF ZOOLOGY ALIGARH 202 002 ALLAHABAD UNIVERSITY ALLAHABAD 211 002 7. Dr. ROSHAN ALAM (1958) C/O MOHD HABIB EDUCATIONAL HOUSE 16. Dr. AMITA MISHRA (2665) AMU SHAM SHAD MARKET C/O SRI RAMESH MISHRA ALIGARH 202 002 G B 53, GANGA SECTOR, TRIVENIPURAM JHUSI , ALLAHABAD 211 019 8. -

Annual Report 2015-2016
Annual Report IISc 2015-16 ANNUAL REPORT IISc 2015-16 www.iiscpress.iisc.in INDIAN INSTITUTE OF SCIENCE VISITOR The President of India PRESIDENT OF THE COURT K Kasturirangan CHAIRMAN OF THE COUNCIL P Rama Rao DIRECTOR Anurag Kumar DEANS SCIENCE: T N Guru Row ENGINEERING: M Narasimha Murty UG PROGRAMME: Umesh Varshney REGISTRAR V Rajarajan IISc ranked India’s top University In 2016, for the first time the NIRF (National Institutional Ranking Framework), under the auspices of the Ministry of Human Resource Development, came out with rankings for Indian Universities and institutions of higher education. Amongst Universities, IISc was ranked Number 1. Contents FOREWORD 2 IISc AT A GLANCE 4 1. The INSTITUTE 14 1.1 Court 1.2 Council 1.3 Finance Committee 1.4 Senate 1.5 Faculties 2. STAFF (ADMINISTRATION) 18 3. DEPARTMENTS/CENTRES/UNITS 21 3.1 Biological Sciences 3.2 Chemical Sciences 3.3 Electrical Sciences 3.4 Interdisciplinary Research 3.5 Mechanical Sciences 3.6 Physical & Mathematical Sciences 3.7 Centres under the Director 4. AWARDS/DISTINCTIONS 230 5. UNDERGRADUATE PROGRAMME 238 6. STUDENTS 240 6.1 Admissions & On Roll 6.2 SC/ST Students 6.3 Scholarships/Fellowships 6.4 Assistance Programme 6.5 Students Council 6.6 Hostels 6.7 Institute Medals 6.8 Awards & Distinctions 6.9 Placement 6.10 External Registration Program 6.11 Research Conferments 7. EVENTS 262 7.1 Institute Lectures 7.2 Conferences/Seminars/Symposia/Workshops 8. OTHER INSTITUTE UNITS 270 8.1 CCMD - Buildings 8.2 Official Language Unit 8.3 SC/ST Cell 8.4 Counselling and Support Centre 8.5 Public Information Office 9.