(Aplocheilus Panchax) ERSS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Genus of Miniature Cynolebiasine from the Atlantic
64 (1): 23 – 33 © Senckenberg Gesellschaft für Naturforschung, 2014. 16.5.2014 A new genus of miniature cynolebiasine from the Atlantic Forest and alternative biogeographical explanations for seasonal killifish distribution patterns in South America (Cyprinodontiformes: Rivulidae) Wilson J. E. M. Costa Laboratório de Sistemática e Evolução de Peixes Teleósteos, Instituto de Biologia, Universidade Federal do Rio de Janeiro, Caixa Postal 68049, CEP 21944 – 970, Rio de Janeiro, Brasil; wcosta(at)acd.ufrj.br Accepted 21.ii.2014. Published online at www.senckenberg.de/vertebrate-zoology on 30.iv.2014. Abstract The analysis of 78 morphological characters for 16 species representing all the lineages of the tribe Cynopoecilini and three out-groups, indicates that the incertae sedis miniature species ‘Leptolebias’ leitaoi Cruz & Peixoto is the sister group of a clade comprising the genera Leptolebias, Campellolebias, and Cynopoecilus, consequently recognised as the only member of a new genus. Mucurilebias gen. nov. is diagnosed by seven autapomorphies: eye occupying great part of head side, low number of caudal-fin rays (21), distal portion of epural much broader than distal portion of parhypural, an oblique red bar through opercle in both sexes, isthmus bright red in males, a white stripe on the distal margin of the dorsal fin in males, and a red stripe on the distal margin of the anal fin in males.Mucurilebias leitaoi is an endangered seasonal species endemic to the Mucuri river basin. The biogeographical analysis of genera of the subfamily Cynolebiasinae using a dispersal-vicariance, event-based parsimony approach indicates that distribution of South American killifishes may be broadly shaped by dispersal events. -

A Replacement Name for the Preoccupied Genus Name Adamas Huber, 1979 (Actinopterygii: Cyprinodontiformes)
_____________Mun. Ent. Zool. Vol. 1, No. 1, January 2006___________ 167 A REPLACEMENT NAME FOR THE PREOCCUPIED GENUS NAME ADAMAS HUBER, 1979 (ACTINOPTERYGII: CYPRINODONTIFORMES) Hüseyin Özdikmen*, Nazmi Polat**, Mahmut Yılmaz*** and Okan Yazıcıoğlu*** * Gazi Üniversitesi, Fen-Edebiyat Fakültesi, Biyoloji Bölümü, 06500 Ankara / TÜRKİYE, e-mail: [email protected] ** Gazi Üniversitesi, Fen-Edebiyat Fakültesi, Biyoloji Bölümü, 06500 Ankara / TÜRKİYE, e-mail: [email protected] *** Gazi Üniversitesi, Fen-Edebiyat Fakültesi, Biyoloji Bölümü, 06500 Ankara / TÜRKİYE, e-mails: [email protected]; [Özdikmen, H., Polat, N., Yılmaz, M. & Yazıcıoğlu, O. 2006. A replacement name for the preoccupied genus name Adamas Huber, 1979 (Actinopterygii: Cyprinodontiformes). Munis Entomology & Zoology, 1 (1): 167-168] ABSTRACT: A replacement name, Fenerbahce is proposed for the genus name Adamas Huber, 1979 in the fish family Aplocheilidae (Cyprinodontiformes). KEY WORDS: Fenerbahce, Adamas, homonymy, replacement name, Actinopterygii, Cyprinodontiformes, Aplocheilidae. Class Actinopterygii Order Cyprinodontiformes Family Aplocheilidae Genus Fenerbahce nom. nov. Adamas Huber, 1979. Journal Am. Killifish Ass. 12 (6): 166 and Revue fr. Aquariol. Herpetol. 6 (1): 6. (Actinopterygii: Cyprinodontiformes: Aplocheiloidei: Aplocheilidae: Aplocheilinae). Preoccupied by Adamas Malaise, 1945. Opusc. ent., Lund, Suppl. 4, 97. (Hymenoptera: Symphyta: Tenthredinoidea: Tenthredinidae: Allantinae: Adamasini). The genus name Adamas was proposed by Malaise, 1945 as an objective replacement name of the genus Dinax Konow, 1897 with the type species Dinax jakowleffi Konow, 1897. For the present, the genus Adamas Malaise, 1945 includes six species (Wei, 2004). Subsequently, the genus Adamas was described by Huber, 1979 with the type species Adamas formosus Huber, 1979 by monotypy from in front of Ntokou village near the banks of Likouala-Mossaka River, Congo. -

Monophyly and Taxonomy of the Neotropical Seasonal Killifish Genus Leptolebias (Teleostei: Aplocheiloidei: Rivulidae), with the Description of a New Genus
Zoological Journal of the Linnean Society, 2008, 153, 147–160. With 11 figures Monophyly and taxonomy of the Neotropical seasonal killifish genus Leptolebias (Teleostei: Aplocheiloidei: Rivulidae), with the description of a new genus WILSON J. E. M. COSTA* Downloaded from https://academic.oup.com/zoolinnean/article/153/1/147/2606377 by guest on 23 November 2020 Laboratório de Ictiologia Geral e Aplicada, Departamento de Zoologia, Universidade Federal do Rio de Janeiro, Caixa Postal 68049, CEP 21944-970, Rio de Janeiro, Brazil Received 30 March 2007; accepted for publication 4 July 2007 A phylogenetic analysis based on morphological characters indicates that Leptolebias Myers, 1952, a genus of small killifishes highly threatened with extinction, from Brazil, is paraphyletic. As a consequence, Leptolebias is restricted in this study to a well-supported clade that includes Leptolebias marmoratus (Ladiges, 1934), Leptolebias splendens (Myers, 1942), Leptolebias opalescens (Myers, 1942), and Leptolebias citrinipinnis (Costa, Lacerda & Tanizaki, 1988), from the coastal plains of Rio de Janeiro, and Leptolebias aureoguttatus (Cruz, 1974) (herein redescribed, and for which a lectotype is designated) and Leptolebias itanhaensis sp. nov., from the coastal plains of São Paulo and Paraná, in southern Brazil. Leptolebias is diagnosed by three synapomorphies: a caudal fin that is longer than deep, a single anterior supraorbital neuromast, and dark pigmentation that does not extend to the distal portion of the dorsal fin in males. A key is provided for the identification of species of Leptolebias. Three species formerly placed in Leptolebias, Leptolebias minimus (Myers, 1942), Leptolebias fractifasciatus (Costa, 1988), and Leptolebias cruzi (Costa, 1988), are transferred to Notholebias gen. -

The Neotropical Genus Austrolebias: an Emerging Model of Annual Killifishes Nibia Berois1, Maria J
lopmen ve ta e l B D io & l l o l g e y C Cell & Developmental Biology Berois, et al., Cell Dev Biol 2014, 3:2 ISSN: 2168-9296 DOI: 10.4172/2168-9296.1000136 Review Article Open Access The Neotropical Genus Austrolebias: An Emerging Model of Annual Killifishes Nibia Berois1, Maria J. Arezo1 and Rafael O. de Sá2* 1Departamento de Biologia Celular y Molecular, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay 2Department of Biology, University of Richmond, Richmond, Virginia, USA *Corresponding author: Rafael O. de Sá, Department of Biology, University of Richmond, Richmond, Virginia, USA, Tel: 804-2898542; Fax: 804-289-8233; E-mail: [email protected] Rec date: Apr 17, 2014; Acc date: May 24, 2014; Pub date: May 27, 2014 Copyright: © 2014 Rafael O. de Sá, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Annual fishes are found in both Africa and South America occupying ephemeral ponds that dried seasonally. Neotropical annual fishes are members of the family Rivulidae that consist of both annual and non-annual fishes. Annual species are characterized by a prolonged embryonic development and a relatively short adult life. Males and females show striking sexual dimorphisms, complex courtship, and mating behaviors. The prolonged embryonic stage has several traits including embryos that are resistant to desiccation and undergo up to three reversible developmental arrests until hatching. These unique developmental adaptations are closely related to the annual fish life cycle and are the key to the survival of the species. -

Draft Risk Assessment Report Nothobranchius Furzeri (Turquoise
APPLICATION TO AMEND THE LIST OF SPECIMENS SUITABLE FOR LIVE IMPORT Draft Risk Assessment Report 1. Taxonomy of the species a. Family name: Aplocheilidae b. Genus name: Nothobranchius c. Species: N. furzeri d. Subspecies: No e. Taxonomic Reference: http://www.uniprot.org/taxonomy/105023 f. Common Names: Turquoise killifish g. Is the species a genetically-modified organism (GMO)? No 2. Status of the species under CITES Species is not listed in the CITES Appendices. 3. Ecology of the species a. Longevity: what is the average lifespan of the species in the wild and in captivity? The average lifespan of the species in captivity is 13 weeks (1). Wild derived N. furzeri from three different habitats showed a maximum lifespan of 25-32 weeks (1). b. What is the maximum length and weight that the species attains? Provide information on the size and weight range for males and females of the species. As per the original formal description of the species, the standard length of N. furzeri from a wild population ranged from 2.5cm to 4.4cm (2). The maximum length the species can attain is 7cm (3). The mean maximal body weight reported for males is 3.8 ± 1.1 g (range: 0.5 – 6.7 g) and 2.2 ± 0.6 g (range: 0.2 - 3.6 g) for females (4). Growth rates of Nothobranchius in the wild have been reported to be similar to those in captivity (5). c. Discuss the identification of the individuals in this species, including if the sexes of the species are readily distinguishable, and if the species is difficult to distinguish from other species. -

01 Astyanax Final Version.Indd
Vertebrate Zoology 59 (1) 2009 31 31 – 40 © Museum für Tierkunde Dresden, ISSN 1864-5755, 29.05.2009 Osteology of the African annual killifi sh genus Callopanchax (Teleostei: Cyprinodontiformes: Nothobranchiidae) and phylogenetic implications WILSON J. E. M. COSTA Laboratório de Ictiologia Geral e Aplicada, Departamento de Zoologia, Universidade Federal do Rio de Janeiro, Caixa Postal 68049, CEP 21944-970, Rio de Janeiro, Brazil E-mail: wcosta(at)acd.ufrj.br Received on May 5, 2008, accepted on October 6, 2008. Published online at www.vertebrate-zoology.de on May 15, 2009. > Abstract Osteological structures of Callopanchax are fi rst described and illustrated. Twenty-six characters derived from comparisons of osseous structures among some aplocheiloid fi shes provided evidence supporting hypotheses of relationships among three western African genera (Callopanchax, Scriptaphyosemion and Archiaphyosemion), as proposed in recent molecular analysis. The clade comprising Callopanchax, Scriptaphyosemion and Archiaphyosemion is supported by a laterally displaced antero-proximal process of the fourth ceratobranchial. The sister group relationship between Callopanchax and Scriptaphyosemion is supported by a constriction on the posterior portion of the parasphenoid, an anterior expansion of the hyomandibula, a rectangular basihyal cartilage, an anterior pointed process on the fi rst vertebra, and a long ventrally directed hemal prezygapophysis on the preural centrum 2. Monophyly of Callopanchax is supported by a convexity on the dorsal margin of the opercle, a long interarcual cartilage, and long neural prezygapophyses on the anterior caudal vertebrae. > Key words Killifi shes, Callopanchax, Africa, Osteology, Annual fi shes. Introduction COSTA, 1998a, 2004) and among genera and species of the Rivulidae (e. g., COSTA, 1998b, 2005, 2006a, b). -

Plio-Pleistocene Phylogeography of the Southeast Asian Blue
RESEARCH ARTICLE Plio-Pleistocene phylogeography of the Southeast Asian Blue Panchax killifish, Aplocheilus panchax Samantha V. Beck1,2*, Gary R. Carvalho3, Axel Barlow4, Lukas Ru¨ ber5,6, Heok Hui Tan7, Estu Nugroho8, Daisy Wowor9, Siti Azizah Mohd Nor10, Fabian Herder11, Zainal A. Muchlisin12, Mark de Bruyn3,13* 1 Ho´lar University College, Department of Aquaculture and Fish Biology, Ha´sko´linn a´ Ho´lum, Sauða´rkro´kur, Iceland, 2 Institute of Life and Environmental Sciences, University of Iceland, Reykjavı´k, Iceland, 3 Molecular Ecology and Fisheries Genetics Laboratory, School of Biological Sciences, Environment Centre Wales, a1111111111 Bangor University, Bangor, United Kingdom, 4 Institute for Biochemistry and Biology, University of Potsdam, a1111111111 Karl-Liebknecht-Strasse, Potsdam (Golm), Germany, 5 Naturhistorisches Museum der Burgergemeinde a1111111111 Bern, Bernastrasse, Bern, Switzerland, 6 Institute of Ecology and Evolution, University of Bern, Baltzerstrasse, Bern, Switzerland, 7 Lee Kong Chian Natural History Museum, National University of a1111111111 Singapore, Singapore, 8 Indonesian Research Institute for Freshwater Aquaculture, Bogor, Java, Indonesia, a1111111111 9 Research Center for Biology (Puslit Biologi), Indonesian Institute of Sciences (LIPI), Cibinong, Indonesia, 10 School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia, 11 Sektion Ichthyologie, Zoologisches Forschungsmuseum Alexander Koenig, Adenauerallee, Bonn, Germany, 12 Department of Aquaculture, Marine & Fishery Sciences, Syiah Kuala University, Banda Aceh, Indonesia, 13 School of Life and Environmental Sciences, University of Sydney, Sydney, New South Wales, Australia OPEN ACCESS * [email protected] (MdB); [email protected] (SVB) Citation: Beck SV, Carvalho GR, Barlow A, Ru¨ber L, Hui Tan H, Nugroho E, et al. (2017) Plio- Pleistocene phylogeography of the Southeast Asian Blue Panchax killifish, Aplocheilus panchax. -

Decline in Fish Species Diversity Due to Climatic and Anthropogenic Factors
Heliyon 7 (2021) e05861 Contents lists available at ScienceDirect Heliyon journal homepage: www.cell.com/heliyon Research article Decline in fish species diversity due to climatic and anthropogenic factors in Hakaluki Haor, an ecologically critical wetland in northeast Bangladesh Md. Saifullah Bin Aziz a, Neaz A. Hasan b, Md. Mostafizur Rahman Mondol a, Md. Mehedi Alam b, Mohammad Mahfujul Haque b,* a Department of Fisheries, University of Rajshahi, Rajshahi, Bangladesh b Department of Aquaculture, Bangladesh Agricultural University, Mymensingh, Bangladesh ARTICLE INFO ABSTRACT Keywords: This study evaluates changes in fish species diversity over time in Hakaluki Haor, an ecologically critical wetland Haor in Bangladesh, and the factors affecting this diversity. Fish species diversity data were collected from fishers using Fish species diversity participatory rural appraisal tools and the change in the fish species diversity was determined using Shannon- Fishers Wiener, Margalef's Richness and Pielou's Evenness indices. Principal component analysis (PCA) was conducted Principal component analysis with a dataset of 150 fishers survey to characterize the major factors responsible for the reduction of fish species Climate change fi Anthropogenic activity diversity. Out of 63 sh species, 83% of them were under the available category in 2008 which decreased to 51% in 2018. Fish species diversity indices for all 12 taxonomic orders in 2008 declined remarkably in 2018. The first PCA (climatic change) responsible for the reduced fish species diversity explained 24.05% of the variance and consisted of erratic rainfall (positive correlation coefficient 0.680), heavy rainfall (À0.544), temperature fluctu- ation (0.561), and beel siltation (0.503). The second PCA was anthropogenic activity, including the use of harmful fishing gear (0.702), application of urea to harvest fish (0.673), drying beels annually (0.531), and overfishing (0.513). -
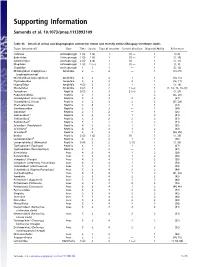
Supporting Information
Supporting Information Samonds et al. 10.1073/pnas.1113993109 Table S1. Details of arrival and biogeographic context for extant and recently extinct Malagasy vertebrate clades Taxon (ancestor of) Class Time Source Type of ancestor Current direction Dispersal Ability References Cichlidae Actinopterygii 1 (3) 1 (2) 1 (1) — 1(1–9) Bedotiidae Actinopterygii 1 (3) 1 (3) 1 (1) — 1 (1, 10) Aplocheilidae Actinopterygii 2 (3) 4 (3) 1 (1) — 1 (1, 11) Mugilidae Actinopterygii 1 (3) 1 (—) 1 (1) — 1 (1, 3) Clupeidae Actinopterygii 1 1 1 — 1 (3, 12) Microhylidae1 (Cophylinae + Amphibia 2 — 2 — 1 (13–15) Scaphiophryninae) Microhylidae2 (Dyscophinae) Amphibia 3 3 2 1 1 (14, 15) Ptychadenidae Amphibia 5 2 2 2 1 (16, 17) Hyperoliidae Amphibia 4 (3) 2 2 1 1 (1, 18) Mantellidae Amphibia 3 (2) 3 2 1 (—) 1 (1, 14, 15, 19–23) Testudinae Reptilia 5 (2) 2 3 2 (—) 2 (1, 24) Podocnemididae Reptilia 2 1 3 — 2 (25, 26) Crocodylidae1 (Crocodylus) Reptilia 5 2 3 2 2 (27) Crocodylidae2 (Voay) Reptilia 5 2 3 2 2 (27, 28) Chamaeleonidae Reptilia 3 4 2 1 1 (29) Gerrhosauridae Reptilia 2 2 2 — 1 (30) Opluridae Reptilia 2 1 2 — 1 (26) Gekkonidae1* Reptilia 3 2 2 1 2 (31) † Gekkonidae2 Reptilia 5 2 2 2 2 (31) ‡ Gekkonidae3 Reptilia 5 2 2 2 2 (31) Scincidae1 (Trachylepis) Reptilia 4 2 2 1 1 (32) Scincidae2§ Reptilia 4 3 3 1 2 (33) { Scincidae3 Reptilia 3 2 2 1 1 (34, 35) Boidae Reptilia 2 (3) 1 (2) 2 (1) — 1 (1, 26) Lamprophiidae1ll Reptilia 4 2 2 1 1 (36) Lamprophiidae2 (Mimophis) Reptilia 5 (4) 2 2 2 (1) 1 (1, 36) Typhlopidae1 (Typhlops) Reptilia 3 1 2 1 1 (37) Typhlopidae2 -

Sexual Dimorphism Characteristics of the White Spot Fish, Aplocheilus Panchax (Hamilton, 1822)
World Applied Sciences Journal 35 (9): 1816-1820, 2017 ISSN 1818-4952 © IDOSI Publications, 2017 DOI: 10.5829/idosi.wasj.2017.1816.1820 Sexual Dimorphism Characteristics of the White Spot Fish, Aplocheilus panchax (Hamilton, 1822) Norshida Ismail, Nur Hanani Mohd Nor, Mohd Khairi Che Lah and Ahmad Syazni Kamarudin Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, 22200, Besut, Terengganu, Malaysia Abstract: Sexual dimorphism characteristics of the white-spot fish, Aplocheilus panchax (Hamilton, 1822) was determined by using the analysis of morphometric and meristic characters, color pattern and gonad observation. Digimizer Analyzer Software was used to measure six morphometric and 21 meristic characters. The characters and Standard Length (SL) were subjected to Independent Sample T-test and Mann-Whitney Test to identify the significant differences between sexes of A. panchax. The findings indicate that SL and Truss 7 (anal fin length) of malesA. panchax are significantly longer than females. The mean SL of males are 34.98±2.98 mm and8.61 mm±0.83 in Truss 7, while females have mean of 33.23 mm±2.55 in SL and 7.47 mm±0.86 in Truss 7. The male gonad (testes) is whitish and female gonad (ovary) is pale color with clearly seen ova in each sac ovary. Meanwhile, the males’ body scales, dorsal, caudal and anal fins reflect a metallic bluish sheen under light which only subtle or absent in females. Black and yellow band border on dorsal fin are more obvious in males than females. Key words: Aplocheilus panchax Ornamental fish Sex determination Fish breeding Morphometric, meristic INTRODUCTION Sexual dimorphism is the differences in body size, shape, coloration or morphology that exhibits by male Aplocheilus panchax(Hamilton, 1822) is a small fish and female of the same species [7]. -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -

Gold Kilifish ( Aplocheilus Lineatus ) Variety
Gold Kilifish ( Aplocheilus lineatus ) Variety Order: Cyprinodontiformes - Family: Aplocheilidae Also known as: Striped Panchax Type: Freshwater Description: The Gold Killifish, is a variety of the species of killifish (Aplocheilus lineatus), , of the genus Aplocheilus. An aquarium variant of this species with a more yellowish coloration is known as golden wonder killifish. The striped panchax inhibits fresh and brackish waters of India and Sri Lanka. It is found in streams, rivers, swamps, and paddy fields. The golden wonder killifish is one of the most readily available killifish in the aquarium industry. It is a good candidate for a first killifish because it is relatively hardy. Physical Characteristics: The golden wonder killifish is yellow and green in color and is one of the bigger killifishes. These fish are best kept in pairs. The golden wonder killifish thrives on plant life, so be sure to include some live plants to keep them happy and healthy. Size / Weight / Age: 6 inches; Up to 15 cm, will spawn at half this size. Color Form: There is a 'Gold' form, commonly known as Golden Wonder, which has become very popular. Sexual dimorphism: Males tend to be larger than females and may have vertical stripes at maturity. Males have stronger colouration and longer, more pointed dorsal and anal fins, while females have a number of vertical black stripes on their rear flanks. Reproduction & Spawning: Lineatus gold is one of the "easier" killifish to keep and breed, and can sometimes be found in area fish stores at outrageous prices. They are usually sold under the name "Golden Wonder Killie" or the less creative "Yellow Killifish." They will eat flake food and are content to occupy the surface of a community tank.